
Acute rhinosinusitis is a common illness that represents a substantial economic burden. Viral infection of the upper respiratory tract is the most common presentation of rhinosinusitis, and the vast majority of cases resolve spontaneously, with only a small proportion developing a secondary bacterial infection that will benefit from antimicrobial therapy. Accurate diagnosis of rhinosinusitis depends upon clinical assessment. Acute bacterial rhinosinusitis is generally diagnosed in the presence of more than 7 to 10 days of nasal discharge. The most common bacterial isolates from acute bacterial rhinosinusitis are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Group A beta-hemolytic streptococci, and Staphylococcus aureus. Anaerobes predominate in rhinosinusitis of dental origin, and fungi and Pseudomonas aeruginosa predominate in rhinosinusitis in neutropenic patients. The proper choice of antibiotic therapy depends on the likely infecting pathogens, bacterial antibiotic resistance, and the antibiotics’ pharmacologic profiles. Isolation of the causative agents should be considered in cases failing initial treatment. In addition to antibiotics, adjuvant therapies and surgery may be utilized in the management of bacterial sinusitis.
A 14-year-old girl presents to the ED on a Friday night with a history of runny nose, sneezing, and sore throat for 12 days. Her previous medical history consists of occasional colds and a few episodes of streptococcal tonsillitis at 7 and 8 years of age, but she is otherwise healthy. She has received all childhood vaccines and has no history of allergy. She has 1 younger sibling, a 7-year-old sister, who is currently healthy but had a sore throat 2 weeks ago, for which she received amoxicillin for 10 days. Her father is a smoker and suffers from chronic bronchitis. He currently has an acute exacerbation of his chronic bronchitis and is being treated with amoxicillin. The patient’s illness started as a cold, with runny nose and fever, and she noticed that other friends at school have also had similar symptoms. She has not taken any medications, but her symptoms have continued, she has had difficulty sleeping at night because of cough, and she has suffered a constant, dull, frontal headache for the past day. She is well developed and cooperative on presentation but seems ill-looking, with a runny nose and constant cough. Her temperature is 37°C (98.6°F). Examination discloses bilateral mucoid nasal discharge, pharyngeal redness, enlarged tonsils, fluid behind the right eardrum without redness, and minimal cervical lymphadenitis. She has no tenderness on percussion or pressure on the cheekbones or frontal bones. The rest of the physical and neurological examinations are normal. What diagnostic tests should you obtain? Should you perform transillumination of the sinuses? Should you obtain radiological studies (ie, plain x-rays or CT)? Is lab work required? Should the patient be referred to an otolaryngologist?
Acute rhinosinusitis is defined as an inflammation of the mucosal lining of the nasal passage and paranasal sinuses lasting up to 4 weeks. Acute rhinosinusitis is one of the most common health problems and has increased in prevalence and incidence,1 causing significant physical symptoms, negatively affecting quality of life, and substantially impairing daily functioning. There are numerous etiologies of the condition, including viral, bacterial, fungal, allergic, and environmental irritants; in addition, many patients develop seemingly idiopathic disease. This issue of Pediatric Emergency Medicine Practice will review the epidemiology and pathophysiology of pediatric rhinosinusitis, review current guidelines differentiating viral and bacterial rhinosinusitis, and offer recommendations on appropriate therapies for treating this common condition.
Several practice guidelines for the treatment of acute bacterial rhinosinusitis (ABRS) have been published in the United States within the past decade,2-7 most recently in 2007 by the American Academy of Otolaryngology – Head and Neck Surgery8 (AAO-HNS) and in 2008 by the Institute for Clinical Systems Improvement.9 These guidelines present varying opinions about the clinical criteria for initiation and choice of empiric antimicrobial regimens. The most recent guideline, developed by The Infectious Diseases Society of America (IDSA),10 addresses some of the more controversial areas concerning initial choice of empiric management of ABRS in both children and adults. Aimed at the community physician or emergency medicine specialist audience, these guidelines focus on the recognition of clinical factors that best distinguish bacterial from viral rhinosinusitis and the selection of the most appropriate antimicrobial agent for treatment, based on evolving antibiotic susceptibility profiles of recent respiratory pathogens in the United States.
Rhinosinusitis is a very common condition, with approximately 24 million cases diagnosed in the United States each year.11,12 It is the fifth leading cause of antimicrobial prescriptions by physicians in office practice, with total direct costs estimated at $3 billion per year.8,10,13,14 A viral upper respiratory infection (URI) is the most common antecedent event. The incidence rates among adults are higher for women than men (approximately 1.7-fold) and children 2 to 6 years of age. Adults between 25 and 64 years are the most commonly affected. Prospective longitudinal studies performed in young children (6-35 months of age) illustrated that viral URIs occurred with an incidence of 6 episodes per patient-year and that 8% (0.5 episodes per patient-year) were complicated by overt acute rhinosinusitis.15
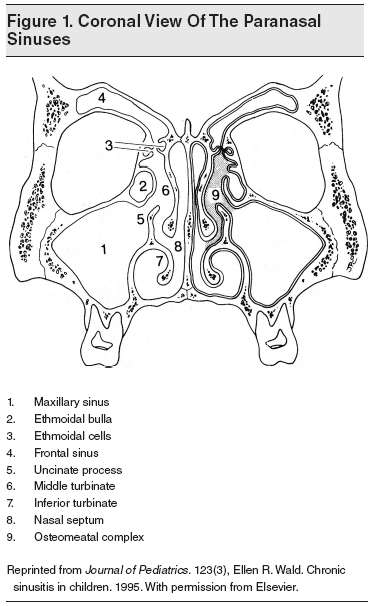
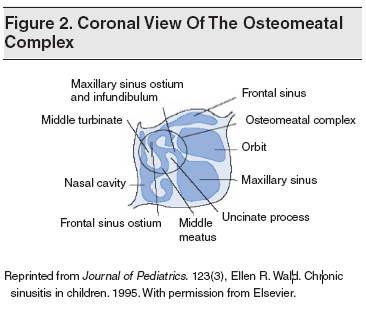
The paranasal sinuses (maxillary, ethmoid, frontal, and sphenoid) include 4 symmetrical air-filled spaces lined by pseudostratified, ciliated, columnar epithelium. They are connected through the sinus ostia, which are small tubular openings that drain into the nasal cavity. (See Figures 1 and 2.) The frontal, anterior ethmoid, and maxillary sinuses drain into the middle meatus, while the posterior ethmoid and sphenoid sinuses open into the superior meatus. The osteomeatal complex (OMC) is the site where the drainage of the frontal, ethmoid, and maxillary sinuses merge. It is bordered medially by the middle turbinate, the basal lamella posteriorly and superiorly, and the lamina papyracea laterally. It opens for drainage anteriorly and inferiorly.
During the third week of embryonic development, proliferation and medial migration of ectodermal cells form the notochord. After the heart tube and pericardium have rotated from the cranial position to lie anteriorly, the notochord (which is initially in the caudal region of the embryonic disc) rotates to lie posterior to the primitive foregut. The paraxial layer of mesenchyme, which lies adjacent to the notochord, differentiates into the somite ridges, intermediate cell mass, and lateral plate mesoderm. From these mesodermal structures, the branchial arches develop, the first of which gives rise to internal nasal structures. (See Table 1.)
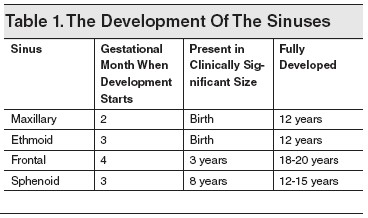
The paranasal sinuses develop in conjunction with the palate from changes in the lateral wall of the nasal cavity. At 40 weeks’ gestation, 2 horizontal grooves develop in the mesenchyme of the lateral wall of the nasal cavity. Proliferation of maxilloturbinate mesenchyme between these grooves results in an outpouching of tissue medially into the nasal lumen. This outpouching is the precursor of the middle and inferior meatus as well as the inferior turbinate. Ethmoidoturbinate folds develop superiorly to give rise to the middle and superior turbinates. Once the turbinate structures are established, sinus development begins and continues until early adulthood.
The sinuses open into the nose via small openings called ostia.1 The maxillary and ethmoid sinuses form at 3 to 4 months’ gestation. Thus, an infant is born with 3 or 4 ethmoid cells and tiny, teardrop-shaped maxillary sinuses. By the teenage years, each maxillary sinus has progressively enlarged to an adult capacity of 15 mL. In healthy individuals, the ethmoid sinuses increase in number to 18 to 20, and each drains by an individual ostium that is 1 to 2 mm in diameter.
The frontal sinus develops from an anterior ethmoid cell and moves to its supraorbital position when the individual is aged 6 to 7 years. Frontal sinuses may begin to develop at this age but usually do not appear radiologically until the individual is approximately 12 years of age. The maxillary, anterior ethmoid, and frontal sinuses drain into the middle meatus; the posterior ethmoid and sphenoid sinuses drain into the superior meatus. Since the infant is born only with the maxillary and ethmoid sinuses, the frontal sinuses are rarely infected prior to 6 years of age.
Rhinosinusitis may be due to 3 pathological causes:
Blockage or inflammation at the OMC is responsible for the development of viral – and subsequently bacterial – sinusitis, as it interferes with effective mucociliary clearance.16 Because the mucous membranes lining the nasal chambers and the sinuses are continuous with each other through the natural ostium and are histologically similar, any URI commonly results in an inflammatory sinusitis; however, in most cases, sinus infection does not persist after the nasal infection has subsided unless there is continued blockage at the osteomeatal complex. At this stage, the blocked sinus does not drain freely and is susceptible to secondary bacterial infection.
Blockage of the sinus ostium is the main predisposing factor for suppurative infection, and it often results from URIs or other viral infections that are common in early childhood. Sinusitis that results from mechanical obstruction can be caused by septal dislocation owing to birth trauma, unilateral choanal atresia, foreign bodies placed in the nose, nasal polyps, tumors, or fractures of the nose. Anatomical variations that narrow the osteomeatal complex, including septal deviation, paradoxical middle turbinates, and Haller cells, make this area more sensitive to obstruction from mucosal inflammation. Up to one-third of cystic fibrosis patients develop polyps, complicating the already abnormal sinus secretions that predispose them to sinusitis.17
The drainage patterns of the paranasal sinuses depend on the mucociliary transport mechanism. The ciliated columnar epithelial cells propel the sinus contents toward the natural sinus ostia, and disruption of their function results in fluid accumulation inside the sinus cavity. Defective ciliary function may result from the loss of ciliated epithelial cells; enhanced airflow; viral, bacterial, or environmental ciliotoxins; inflammatory mediators; scars; and Kartagener syndrome.18 Ciliary function is also decreased in the presence of low pH, cold air, hypoxia, dehydration, direct or indirect exposure to cigarette smoke, chemical toxins, bacterial toxins, viral infection, and drugs (eg, anticholinergic medications and antihistamines).
The sinonasal secretions play a major role in the pathophysiology of rhinosinusitis. The mucus blanket that lines the paranasal sinuses contains immunoglobulins, mucoglycoproteins, and inflammatory cells.18 The mucus blanket possesses 2 layers: (1) an inner serous layer (sol phase), and (2) an outer, more viscous layer (gel phase), which is transported by the ciliary beat. Adequate balance between these layers is essential for proper mucociliary clearance.
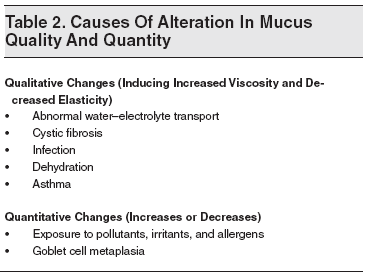
An increase in the mucosal viscosity, as in cystic fibrosis, slows the transport toward the ostia and the gel layer thickens, causing retention of thick mucus inside the sinus. In the absence of secretions or a loss of humidity, the mucus becomes increasingly viscous and the sol phase may become very thin, allowing the gel phase to have intense contact with the cilia and thus impeding their action. Overproduction of mucus can overwhelm the mucociliary clearance system, resulting in retained secretions inside the sinuses. The causes of alteration in mucus quality and quantity are summarized in Table 2. Other contributory factors are asthma,19 acquired immune deficiencies,20 cyanotic congenital heart disease, and dental infections.21 Dental infections can extend into the maxillary sinus cavity from the molar and premolar teeth. The conditions that predispose to ABRS are summarized in Table 3.

The most common bacteria recovered from pediatric and adult patients with community-acquired ABRS are Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Group A beta-hemolytic streptococci, and Staphylococcus aureus.21-26 (See Table 4.) The last time the microbiology of ABRS in children was studied was in 1984.26 The vaccination of children with the 7-valent pneumococcal vaccine, introduced in 2000 in the United States, brought about the decline in the recovery rate of S pneumoniae and an increase in H influenzae.27

The microbiology of ABRS in children can be extrapolated from studies of middle ear fluid of children with acute otitis media in the postpneumococcal vaccine era.28 The predominant respiratory pathogens were S pneumoniae, nontypeable H influenzae, and M catarrhalis; however, the proportion of H influenzae in relation to S pneumoniae has increased in recent years,27 and it has become the predominant isolate.29
S aureus is a common pathogen in sphenoid sinusitis.22 Recent data illustrate a significant increase in the rate of recovery of methicillin-resistant S aureus (MRSA) in patients with upper respiratory tract infections,30 including acute and chronic maxillary rhinosinusitis.31
ARBS is polymicrobial in about one-third of patients. Enteric bacteria are rarely isolated, and anaerobes are isolated only from a few cases of ABRS; however, proper methods for their recovery were rarely employed in most studies of ABRS. Anaerobic bacteria account for about 8% of isolates and are often recovered from ABRS associated with an odontogenic origin, mainly as an extension of the infection from the roots of the premolar or molar teeth.21,32
Odontogenic rhinosinusitis accounts for about 10% to 12% of cases of maxillary rhinosinusitis. Brook evaluated the microbiology of 20 patients with acute maxillary rhinosinusitis associated with odontogenic infection.21 Polymicrobial infection was common with 3.4 isolates per specimen, and 90% of the isolates were anaerobes. The predominant anaerobes were pigmented Prevotella and Porphyromas, Peptostreptococcus, and Fusobacterium species. The predominant aerobes were alpha-hemolytic streptococci, microaerophilic streptococci, and S aureus. Pseudomonas aeruginosa and other aerobic and facultative Gram-negative rods are mostly recovered from patients with nosocomial rhinosinusitis (mainly in those with nasal tubes or catheters), the immunocompromised, those with HIV infection,33 and those with cystic fibrosis.34
The microorganisms recovered from odontogenic infections generally reflect the indigenous oral microflora. An association between periapical abscesses and rhinosinusitis was established in a study of purulent aspirates from 5 periapical abscesses of the upper jaw and their corresponding maxillary sinuses.32 Polymicrobial flora were present in all cases where the number of isolates varied from 2 to 5. Anaerobes were recovered from all specimens. Concordance in the microbiological findings between periapical abscess and the maxillary sinus flora was evident in all instances, suggesting contiguous spread of infection from the periapical abscess. The proximity of the maxillary molar teeth to the floor of the maxillary sinus enables the spread.
The origin of the pathogens introduced into the sinuses that eventually cause rhinosinusitis is, primarily, the nasal cavity. The normal flora of the nasal cavity contain certain bacterial species and include S aureus, Staphylococcus epidermidis, alpha- and gamma-streptococci, Propionibacterium acnes, and facultative diphtheroids.35,36 Potential sinus pathogens are rarely found in the healthy nasal cavity.
The chronology of sinusitis (as well as otitis media) progresses through several phases. (See Figure 3.) The early phase is generally viral (mostly rhinovirus, adenovirus, influenza, and parainfluenza viruses) and usually lasts up to 10 days. Complete recovery occurs in 99% of individuals.37 In a small number of patients, a secondary acute bacterial infection may emerge, generally caused by aerobic bacteria (ie, S pneumoniae, H influenzae, or M catarrhalis). If resolution does not take place, anaerobic bacteria from the oropharyngeal flora become predominant over time.38 The mechanism by which viruses predispose to bacterial sinusitis may involve viral-bacterial synergy, induction of local inflammation that blocks the sinus ostia, increase of bacterial attachment to the epithelial cells, and disruption of the local immune defense.
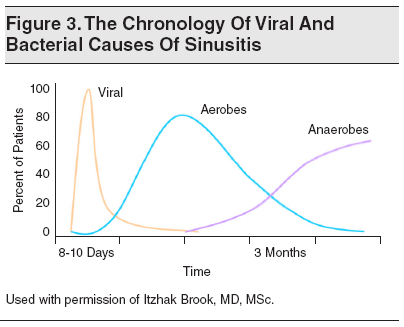
Conditions that promote the growth of anaerobic bacteria include reduction in oxygen tension and an increase in acidity within the sinus. These are caused by the persistent edema and swelling that decrease blood supply and by the consumption of oxygen by the aerobic bacteria.39 Another explanation for the slower appearance of anaerobes as pathogens is that expression of some of their virulence factors, such as a capsule, is slow.40 Several anaerobic and aerobic bacteria that are part of the normal oropharyngeal flora can interfere with the growth of sinus pathogens. Interfering organisms were recovered in higher numbers in the nasopharynx of individuals who are not prone to rhinosinusitis, as compared to individuals who are prone to rhinosinusitis.41
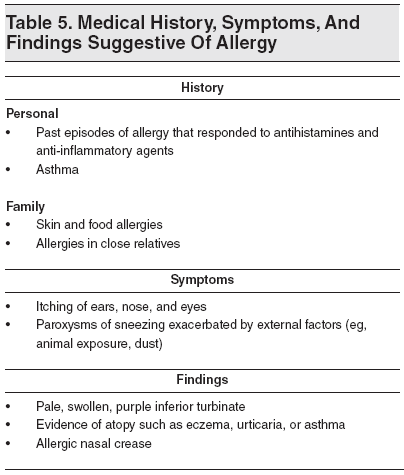
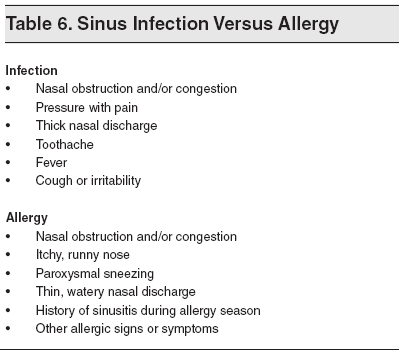
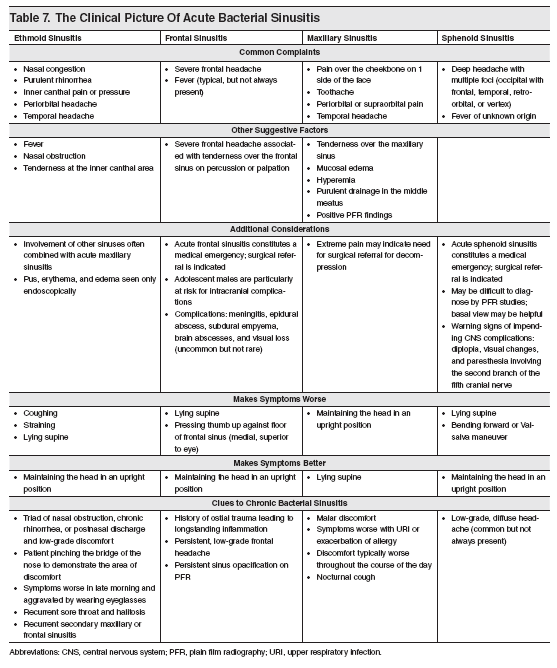
The patient’s medical history should be obtained from the caregiver and, if possible, from the child. It should include inquiry about previous episodes of sinusitis and other upper and lower respiratory tract infections, previous use of antibiotics, the possibility of nasal foreign bodies (especially in children younger than 5 years), attendance at day care centers, immunizations (including against S pneumoniae), history of allergy (see Tables 5 and 6), exposure to cigarette smoke, comorbidities, and previous hospitalization. The presence of any swelling and pain, especially in the facial, forehead, temporal, or orbital areas, or any other site in the head, should be noted. Information about what makes the symptoms worse or better should also be obtained. (See Table 7.)
It is essential to document the length of symptoms of the current respiratory tract infection and symptoms such as cough, nasal secretions, headaches, pain, fever, hyposmia, and/or dental pain or problems. Noting the duration of symptoms can assist in the diagnosis of bacterial sinusitis.
The physical examination should include the following:
Note: transillumination is infrequently utilized because the findings do not always correlate with the disorder. The poor reproducibility between observers limits the use of this method.
Indications for referral to an otolaryngologist for maxillary sinus aspiration include: (1) failure to improve on antimicrobial therapy, (2) severe facial pain, (3) orbital or intracranial complications, and (4) an immunocompromised host (because of the unique microbiology).
Signs of sinus infection that can be observed by physical examination include:
Suspicion of ABRS is based on clinical symptoms and signs when at least 2 major criteria or 1 major and 2 minor criteria are present.42 (See Table 8.) The most common presentation is a persistent (and nonimproved) nasal discharge or cough (or both) lasting more than 10 days.43 Typical clinical manifestations of bacterial sinusitis in children are cough (80%), nasal discharge (76%), and fever (63%). In children, malodorous, fetid breath is common while facial pain, swelling, and headache are rare. There is only one study of children that has correlated the presence of respiratory signs and symptoms with the findings of sinus aspiration. Wald et al performed sinus puncture in children with either “persistent” symptoms or “severe” disease and recovered significant pathogens in high density in 75% of the patients.44

Differentiating viral URI from ABRS is critical and remains quite difficult. The hallmark of viral URIs is the presence of nasal symptoms (discharge and congestion/obstruction) and/or cough and sometimes a scratchy throat. Fever is absent in most patients; when present, it occurs early in the illness. Fever and constitutional symptoms generally disappear within 24 to 48 hours, after which the respiratory symptoms predominate. Generally, the nasal discharge is initially clear and watery but, often, its quality changes over time. In most individuals, the discharge turns thicker and more mucoid and sometimes purulent.35 After a few days, these changes are reversed, with the purulent discharge becoming mucoid and then clear or dry. These changes happen in uncomplicated viral URIs without the use of antimicrobial agents.
The time course of viral URI is the most important characteristic that differentiates it from ABRS. Generally, respiratory symptoms of uncomplicated viral URI last 5 to 10 days. Even though the symptoms do not completely disappear on the tenth day, most respiratory symptoms peak by days 3 to 6 and start to improve afterward. The characteristic presenting symptoms that are commonly associated with a bacterial rather than viral infection have been evaluated by 5 consensus panels created by 5 national societies.45-47 The panels highlighted 3 clinical presentations that should prompt consideration of ABRS rather than a viral URI:

The European Position Paper on Rhinosinusitis and Nasal Polyps, published in 2007, generated a similar definition for ABRS (ie, “worsening” of symptoms after 5 days or persistent symptoms after 10 days with less than 12 weeks duration).52 A 10-day period of symptoms generally differentiates simple viral URI and bacterial sinusitis. Most uncomplicated viral URIs last between 5 and 7 days, and most patients have improved by day 10. The symptoms and signs of acute bacterial infection can be divided into nonsevere and severe.52 (See Table 9.) The severe form carries a higher risk of complications and mandates earlier use of antimicrobial therapy. The combination of high fever and purulent nasal discharge that lasts for at least 3 to 4 days suggests ABRS.

Individuals with ABRS often have nasal edema of the mucous membranes, mucopurulent nasal discharge, persistent postnasal drip, fever, and malaise. The quality of the nasal discharge varies, and it can be thin or thick, clear, mucoid, or purulent. Tenderness and pain of the involved sinus can be induced by percussion of the affected sinus. Cellulitis can also be present, overlying the affected sinus. Other findings, especially in acute ethmoiditis, are periorbital cellulitis, edema, and proptosis. Failure to transilluminate the sinus and a “nasal” sound to the voice can be present in many patients. Direct smear of nasal secretions usually shows the predominance of neutrophils, and the observation of numerous eosinophils suggests allergy.
The symptoms are generally protracted and vary considerably in subacute or chronic bacterial sinusitis. Fever can be of low grade or it can be absent. The patient may complain of malaise, easy fatigability, irregular nasal or postnasal discharge, frequent headaches, difficulty in mental concentration, anorexia, and pain or tenderness to palpation over the affected sinus. Cough and nasal congestion can persist, and a sore throat is frequent due to mouth breathing.
The location of the facial pain can point to the involved sinus. Maxillary rhinosinusitis is commonly associated with pain in the cheeks, frontal sinusitis with the forehead, ethmoid sinusitis with medial canthus, and sphenoid sinusitis with occipital pain. In patients with chronic infection, changes in motion or position can worsen or alleviate the sinus symptoms. (See Table 7.) Pathology in the upper molar teeth can be the source of maxillary rhinosinusitis.
The gold standard for the diagnosis of ABRS is the isolation of bacteria in high density (≥ 104 colony-forming units/mL) from the paranasal sinus cavity. Improper decontamination of the paranasal mucosa prior to aspiration or quantification of the bacteria may lead to misinterpretation of results.44,53-55 Sinus aspiration is an invasive and painful procedure that is impractical in the office or ED setting. Endoscopically guided middle meatus cultures can be used as a surrogate for sinus aspirates in patients with ABRS.56 Nonetheless, obtaining such cultures is beyond the scope of most emergency clinicians, and its validity in children has not been well-established. Therefore, the diagnosis of ABRS in most randomized controlled trials is based on the presence of compatible symptoms and signs, with radiographic confirmation of sinus involvement. (See Table 8.) Unfortunately, these diagnostic criteria do not adequately differentiate between bacterial and viral sinusitis, leading to administration of antimicrobial therapy to individuals who do not require it.
Imaging studies, such as plain radiographs or computed tomography (CT), are often utilized for the diagnosis of ABRS; however, these are nonspecific and cannot differentiate viral from bacterial rhinosinusitis. Sinus CTs are often abnormal in healthy children57,58 and those having CT for nonrespiratory reasons.59 In one study, more than half of the children with viral URI had abnormal maxillary sinus radiographs.57 Another study of CT performed on young adults recovering from colds illustrated that 87% of the subjects had significant abnormalities of their maxillary sinuses.60 Other studies on magnetic resonance imaging (MRI) illustrated that 68% of symptomatic children with acute respiratory infection61 and 42% of healthy children62 had significant sinus abnormalities.
These studies indicate that imaging studies in most children with uncomplicated viral URI will show major abnormalities that are indistinguishable from those associated with bacterial sinusitis. Therefore, imaging studies can only be useful when they are negative, as they confirm the absence of ABRS. Abnormal radiographic studies cannot assist in the diagnosis of ABRS, and such studies are not required in children with uncomplicated ABRS. Most patients with ABRS do not require radiographic evaluation. Imaging is helpful in determining the disease location and extent beyond the site of the original source. These studies may occasionally help in supporting the diagnosis or determine the degree of mucosal involvement, guiding a more effective approach to therapy.63
In patients with ABRS suspected of having suppurative complications, axial and coronal views of contrast-enhanced CT, rather than MRI, are recommended for localization of infection and to guide further treatment. CT or MRI should generally be performed only in those with recurrent or complicated sinusitis or when suppurative complications are suspected. Suppurative complications of ABRS are infrequent, occurring in 3.7% to 11% of hospitalized children with sinusitis. These are mostly associated with potential orbital and intracranial complications of sinusitis.64 CT is considered best for the assessment of bony and anatomical changes associated with sinusitis. It is also helpful in surgical planning and for intraoperative image-guided navigation. MRI is not associated with exposure to ionizing radiation and is most effective in evaluating the extent of soft-tissue inflammation and abnormalities.65-67
In 2002, the diagnostic accuracy of clinical assessment compared to CT or MRI in the diagnosis of orbital and intracranial complications arising from sinusitis and confirmed by intraoperative findings was evaluated in 82 adults and children.68 The diagnostic accuracy was 82% by clinical assessment and 91% by CT imaging in the 43 patients with orbital infections. In the 39 patients with intracranial infections, the diagnostic accuracy was 82% by clinical assessment, 87% by CT, and 97% by MRI. MRI seems, therefore, to be more sensitive than CT in detecting soft-tissue involvement in those with suspected intracranial complications.69-71 In a 2006 study, nonenhanced CT missed the diagnosis of intracranial empyema associated with sinusitis in 4 of 12 children.71 Axial imaging alone failed to demonstrate the empyema in 1 child with sphenoidal and ethmoid sinusitis, while coronal imaging detected its presence and extent. Using contrast-enhanced CT or MRI, the empyema was evident in all the children.
The American College of Radiology has recently developed criteria for the adequacy of imaging examinations for acute rhinosinusitis in children.72 These criteria concluded that both CT and MRI are complementary for the evaluation of suspected orbital and/or intracranial complication of sinusitis. However, the IDSA panel favored contrast-enhanced CT over MRI because of its greater value and relative availability and speed as well as the lack of the need for sedation (which is frequently required for MRI studies in infants and children).10 CT is especially advantageous in children because their sinuses are often asymmetrical and smaller than those in adults.73
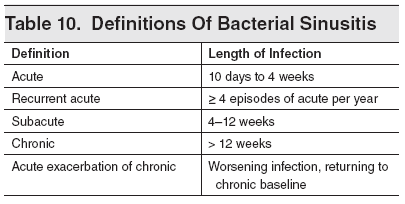
Because establishing a diagnosis may be difficult, differentiation must be made between allergic rhinitis, other causes of head or facial pain, asthma, and dental disorders. An allergic etiology can be confirmed by establishing a history of nasal symptoms and a history of allergy.52 (See Tables 5 and 6.) ABRS has to be differentiated chronologically from other types of rhinosinusitis. These definitions include recurrent acute, subacute, chronic, and acute exacerbation of chronic rhinosinusitis. (See Table 10.)


The symptoms and signs of acute bacterial sinusitis can be divided into nonsevere and severe forms.52 (See Table 9.) The severe form carries a higher risk of complications and mandates earlier use of antimicrobial therapy. The combination of high fever and purulent nasal discharge lasting for at least 3 to 4 days points to a bacterial infection of the sinuses. In children with subacute or chronic bacterial sinusitis, the symptoms are protracted. Fever is rare, the cough and nasal congestion persist, and a sore throat (as a result of mouth breathing) is common.

The medical management of ABRS includes the use of antibiotics and adjuvant therapy. The primary goals of therapy are to:
The management of sinusitis has become a challenging endeavor. The choice of appropriate antimicrobial agents has become more complex in recent years because many of the predominant bacterial pathogens have developed resistance to commonly used antibiotics, which has increased consistently over time.74 The major mechanism of resistance to beta-lactam antibiotics are:
The production of the enzyme, beta-lactamase, is one of the important mechanisms of penicillin resistance. The predominant beta-lactamase-producing bacteria (BLPB) in acute sinusitis are H influenzae and M catarrhalis. Critchley et al reported that during 2005-2006 in the United States, 91.6% of clinical isolates of M catarrhalis and 27.4% of H influenzae were beta-lactamase positive. Regional differences were noted in the prevalence of beta-lactamase production among H influenzae isolates, from 35% in the southeastern states to 25% in the southwestern states.74
BLPB can protect penicillin-susceptible organisms from the activity of penicillin, thereby contributing to their persistence.75 The ability of BLPB to shield penicillin-sensitive organisms has been demonstrated in vitro and in vivo.76 The actual activity of beta-lactamase and the phenomenon of “shielding” have been demonstrated in acutely and chronically inflamed sinus fluids.77 BLPB were isolated in sinus aspirates from 4 of 10 patients with acute sinusitis and 10 of 13 patients with chronic sinusitis.77
The presence of BLPB in sinusitis is not surprising, since over two-thirds of the patients with acute rhinosinusitis and all of the patients with chronic rhinosinusitis received antimicrobial agents that might have selected for BLPB.77 It is therefore plausible that, whenever BLPB are present, therapy needs to be directed at their eradication.
S pneumoniae is resistant to penicillins through changes in penicillin-binding proteins.78 The national rate of resistance has increased steadily in the past decade and is close to 35% of all isolates. Approximately one-half of the penicillin-resistant strains are currently intermediately resistant, and the remainder are highly resistant.
Appropriate antibiotic therapy is essential for the prevention of complications.2 Cultures obtained through direct aspiration or endoscopy can direct the selection of antimicrobials in the treatment of patients who fail to respond.38 This was demonstrated when serial cultures of sinus aspirates were obtained from patients who failed to respond to antimicrobials. Most of the bacteria isolated from the first culture were aerobic or facultative bacteria: S pneumoniae, H influenzae nontype-b, and M catarrhalis. Subsequent sinus cultures of those who failed therapy generally yielded organisms that were resistant to the antimicrobial agents prescribed for treatment. Failure to improve was associated with the emergence of resistant aerobic and anaerobic bacteria in subsequent sinus aspirates. The infection was eradicated in all individuals after the administration of antimicrobials effective against these bacteria and in several instances by surgical drainage.38
The emerging antimicrobial resistance among respiratory infection pathogens leads to the empirical overutilization of broad-spectrum antibiotics, which generates selective pressure that promotes the emergence of greater antimicrobial resistance.79,80 This phenomenon is further aggravated by the paucity of adequate studies to confirm the bacterial etiology and document the response to antimicrobial therapy.
Even though there is definitive evidence that antimicrobial resistance may be an important cause of treatment failure in ABRS,81 the in vitro determination of antimicrobial resistance may not necessarily correlate with poor clinical outcome. This raises the question of whether the emergence of antimicrobial resistance should be the main determinant in selecting the initial empiric antimicrobial therapy for ABRS or whether it should be considered mainly for those who have failed initial therapy to first-line agents (especially those who are seriously ill, immunocompromised, or at risk for severe complications such as orbital or intracranial extension of infection).
Empiric antimicrobial therapy should be started as soon as the clinical diagnosis of ABRS is made. Pharmacokinetic/pharmacodynamic (PK/PD) principles should guide adequate dosing for respiratory tract infections.82 The adoption of more strict criteria for diagnosing ABRS negates withholding or delaying empiric antimicrobial therapy. The utility of these diagnostic criteria for initiating antibiotic treatment has been validated by 3 randomized clinical trials in pediatric patients.83-85 Eligibility in 2 of the studies83,84 was for those presenting with “persistent” respiratory symptoms ≥ 10 days. Only patients with “persistent,” “severe,” and “worsening” presentations were evaluated in the other study.85 These studies demonstrated significantly higher cure rates in those treated with antibiotics compared to placebo (mean 78% vs 60%; odds ratio [OR] 2.52 [95% confidence interval (CI), 1.52-4.18]).
Some children with mild, but persistent, symptoms can be observed for 3 days without giving antimicrobial therapy because 84% of clinical failures happen within 3 days in children receiving placebo.85 These children need close observation, and antimicrobials should be given if improvement has not occurred within 3 days.
Amoxicillin is no longer considered to be adequate for the initial empiric treatment of ABRS in children.10 The addition of clavulanate would improve the coverage of amoxicillin against many beta-lactamase-producing respiratory pathogens in ABRS, which are estimated to be present in about a quarter of all patients. These include approximately 25% to 35% of H influenzae and 90% of M catarrhalis cases.86
The standard dose of amoxicillin-clavulanate is 500 mg by mouth (PO) 3 times per day or 45 mg/kg/day PO 3 times per day or 2 times per day. The high dose of amoxicillin-clavulanate is 2 g PO 2 times per day or 90 mg/kg/day PO 2 times per day. The main disadvantages of using the high-dose amoxicillin-clavulanate are the added cost and potentially greater incidence of adverse effects. High-dose amoxicillin-clavulanate is recommended for children with ABRS from geographic locations with high endemic rates of penicillin-nonsusceptible S pneumoniae as well as for children who have been recently hospitalized, have used an antibiotic within the past month, have severe infection with evidence of systemic toxicity (eg, fever of ≥ 39°C [≥ 102.2°F]), have comorbidities, or are immunocompromised.
High-dose amoxicillin is preferred over standard-dose amoxicillin mainly to enable coverage against penicillin-nonsusceptible S pneumoniae and the less common occurrence of ampicillin-resistant nonbeta-lactamase-producing H influenzae.86 There is only indirect support for the use of high-dose amoxicillin-clavulanate for initial empiric management of ABRS in high-risk patients because these individuals are either at risk for resistant pathogens or may have a poor outcome from treatment failure.87,88
The frequency of penicillin-nonsusceptible S pneumoniae in the United States varies, but it was highest in the southeastern states (approximately 25%) and lowest in the northwestern states (approximately 9%) during 2005-2006.74 The prevalence of resistant or intermediate S pneumoniae in a given community may vary not only geographically but also temporally. This is evidenced by the temporal shift in S pneumoniae susceptibility profiles in some communities following the introduction of pneumococcal conjugate vaccines, resulting in the subsequent emergence of highly virulent and resistant nonvaccine serotypes.28,89 Therefore, decisions concerning appropriate dosing regimens should be based upon susceptibility profiles of prevalent pathogens established by surveillance studies.
The rate of resistance is influenced by recent antimicrobial use, day care attendance, and age < 2 years. Studies determining S pneumoniae penicillin resistance using the revised Clinical Laboratory Standards Institute (CLSI) breakpoints defining penicillin-intermediate (minimum inhibitory concentration [MIC] 4 micg/mL, treatable with high-dose amoxicillin) and penicillin-resistant S pneumoniae (MIC ≥ 8 micg/mL, untreatable with amoxicillin), illustrated a higher rate of penicillin susceptibility (89%-93%).74,86,90,91 These findings suggest that unless the rate of penicillin-nonsusceptible S pneumoniae in the community is high (> 10%), standard-dose amoxicillin-clavulanate should be adequate for the treatment of nonmeningitic S pneumoniae infections, including ABRS.
Oral cephalosporins are inactive against penicillin-resistant S pneumoniae.92,93 The activity of second- and third-generation oral cephalosporins (such as cefaclor, cefuroxime axetil, cefpodoxime, cefprozil, cefdinir, and cefixime) is variable against penicillin-intermediate and penicillin-resistant S pneumoniae. Cefpodoxime, cefuroxime axetil, and cefdinir are moderately active against this organism (< 50% susceptible), cefixime is less effective, and cefaclor and cefprozil are inactive.86,92-94 The parenteral third-generation cephalosporins, cefotaxime and ceftriaxone, are active against all S pneumoniae, including penicillin-resistant ones, and are the recommended second-line empiric therapy (in place of high-dose amoxicillin-clavulanate) for hospitalized children. The most active oral cephalosporin against both H influenzae and M catarrhalis (beta-lactamase positive and negative) is cefpodoxime, followed by cefixime, cefuroxime, and cefdinir.92,95 Cefaclor and cefprozil are least active.
It is evident that there is significant variability in the activity of second- and third-generation oral cephalosporins against S pneumoniae and H influenzae. These agents are, therefore, no longer adequate as monotherapy for the initial empiric treatment of ABRS in children. If an oral cephalosporin is to be used, a third-generation cephalosporin (eg, cefpodoxime or cefixime) combined with clindamycin is recommended in regions with high isolation rates of penicillin-nonsusceptible S pneumoniae (≥ 10% using 2008 CLSI revised breakpoints).
The increased recovery of MRSA in ABRS requires consideration of the need for coverage against these organisms.97 A comparison of the rate of recovery of MRSA between 2001-2003 and 2004-2006 in 244 patients with ABRS illustrated a significant increase in the rate of recovery of this organism in patients. Between 2001 and 2003, S aureus was isolated from 10 (8%) of the patients with acute sinusitis, 3 of which (30%) were MRSA. Between 2004 and 2006, S aureus was isolated from 13 (10%) of the patients with acute sinusitis, 9 of which (69%) were MRSA (P < 0.01).31 This finding suggests that clinicians should have a greater index of suspicion for the presence of MRSA in sinusitis and should make greater use of sinus cultures, especially in patients who do not improve or fail antimicrobial therapy after 48 hours of therapy. Since the nose is a well-known reservoir for S aureus, there remains a concern that, in some instances, the recovery of S aureus (including MRSA) could be due to contamination by the nasal flora during sinus aspiration or acquisition of middle meatus cultures. Accurate diagnosis of MRSA rhinosinusitis with microbiological confirmation is essential for appropriate antimicrobial treatment.
Currently, there is insufficient evidence to support empiric coverage for MRSA in the therapy of ABRS. Nonetheless, in seriously ill patients with clinical manifestations suggestive of orbital or intracranial extension of infection and hospitalized patients with nosocomial sinusitis associated with prolonged nasal intubation, empiric coverage for MRSA while awaiting confirmation from the sinus or middle meatus cultures is helpful.
Treatment of sinus infection associated with the recovery of MRSA is challenging. It is important to provide coverage against these organisms as well as other potential pathogens. Although vancomycin is considered the gold standard for therapy of MRSA infections, reports of increasing in vitro resistance to vancomycin97 combined with reports of clinical failures (with this and other antistaphylococcal agents) underscore the need for alternative therapies.98 Older agents with favorable in vitro activity available in both oral and intravenous (IV) dose forms include trimethoprim-sulfamethoxazole (TMP/SMX) and clindamycin. Minimal clinical data exist to support their routine use as initial therapy in the treatment of MRSA infections. Newer treatment options of therapies for MRSA include linezolid, quinupristin-dalfopristin, daptomycin, and tigecycline.
Alternative agents for empiric initial antimicrobial therapy for those allergic to penicillin may be warranted. For children with a history of immediate-type hypersensitivity response, levofloxacin is recommended as an alternative to amoxicillin-clavulanate. In patients with a history of nontype I hypersensitivity reaction to penicillin, a third-generation oral cephalosporin (eg, cefixime or cefpodoxime) in combination with clindamycin is recommended. Cefixime or cefpodoxime are active against most strains of H influenzae and M catarrhalis, while clindamycin is active against S pneumoniae, including penicillin-intermediate and penicillin-resistant strains.86
The current treatment guidelines for ABRS that generally recommend a course of antimicrobial therapy for 10 to 14 days are based upon the duration of therapy in many of the randomized controlled studies in adults.48 Some have recommended that treatment be continued for 7 days beyond the time symptoms have resolved.99 Data about the optimal duration of therapy in pediatric patients are not conclusive because the efficacy of shorter courses of therapy has not been studied in a rigorous, randomized fashion.100 In children with ABRS, the longer treatment duration of 10 to 14 days is still recommended.10 An alternative therapeutic approach is needed if symptoms worsen after 3 days or fail to improve despite 3 to 5 days of initial empiric antimicrobial therapy.
Individuals with ABRS should start to clinically improve in 3 to 5 days following initiation of effective antimicrobials.85 In one study, complete resolution of respiratory symptoms occurred in 45% of children with ABRS on antibiotics compared to 11% of those on placebo.84 A study that compared high-dose amoxicillin-clavulanate to placebo illustrated that 83% of the 23 children (19 in the placebo group and 4 in the antibiotic group) who failed to improve or worsened did so within 3 days.85
Bacteriological eradication studies also illustrate that most pathogens are eliminated from the maxillary sinuses by the third day of adequate antimicrobial therapy.101-105 Correlation was noted between time to bacterial eradication and time to clinical resolution.102 At 3 days following the initiation of therapy, all pathogens were eradicated from 87.5% patients and all signs and symptoms had improved in 81%; by 5 days, all signs and symptoms were completely resolved in 88%. Thus, a bacteriologic as well as clinical response may be expected within 3 to 5 days in the majority of patients receiving adequate antimicrobial treatment. Children who clinically worsen despite 3 days of empiric antimicrobial therapy should be investigated for the possible causes of failure. These include infection with resistant pathogens, inadequate dosing, and noninfectious causes such as allergy and structural abnormalities. Similarly, if there is no clinical improvement within 3 to 5 days despite initial empiric antimicrobial therapy, an alternate management strategy should be considered even if there is no clinical worsening. Clinical judgment and adequate monitoring is critical in determining whether there is treatment failure or just a slow clinical response.
In one study, consecutive endoscopic cultures from maxillary sinuses were performed on aspirates obtained from 20 patients with ABRS who failed initial empiric antimicrobial therapy.41 An increased level of resistance, with MIC at least 2 times higher as for the pretreatment isolate, was identified in half of patients. This study illustrates that bacterial resistance should be considered in all patients who fail to respond to initial empiric antimicrobial therapy.
In choosing a second-line regimen in a patient who has failed initial antimicrobial therapy, an agent with a broader spectrum of activity and in a different antimicrobial class should be considered.106,107 Antimicrobials selected should be active against penicillin-nonsusceptible S pneumoniae and ampicillin-resistant H influenzae as well as other beta-lactamase-producing respiratory pathogens.
The recommended second-line antimicrobial agents suitable for children with treatment failure to first-line agents are amoxicillin-clavulanate (90 mg/kg/day PO, 2 times per day) and clindamycin (30-40 mg/kg/day PO, 3 times per day) plus cefixime (8 mg/kg/day PO, 2 times per day) or cefpodoxime (10 mg/kg/day PO, 2 times per day).
In patients who have failed to respond to empiric antimicrobial therapy, it is advisable to obtain cultures from the involved sinuses. Identification and susceptibility testing of the isolated pathogens can guide the choice of the second-line agent(s). Endoscopically guided middle meatus cultures can be considered as an alternative in adults108; however, their reliability in children has not been established. Maxillary sinus puncture is recommended to identity potential pathogens and their antimicrobial susceptibility profile in children whose endoscopic cultures show no growth. Nasopharyngeal cultures are unreliable and are not recommended for the microbiologic diagnosis of ABRS.56
In addition to antibiotics, other therapies have been utilized in the management of bacterial sinusitis. These therapies included topical and systemic decongestants, corticosteroids, anti-inflammatory agents, mucolytic agents, humidification, antihistamines, nasal irrigation, saline nasal spray, spicy food, and hot, dry air.109 These agents induce rapid vasoconstriction, improve ostial potency, reduce swelling and congestion of the turbinates, and decrease inflammation at the osteomeatal, thus facilitating sinus drainage. Use of any intranasal medications in children may not be tolerated well.
Neither topical nor oral decongestants and/or antihistamines are recommended as adjunctive treatment in patients with ABRS. Topical decongestants may induce rebound congestion and inflammation, while oral antihistamines may induce drowsiness, xerostomia, and other adverse effects. Even though decongestants and antihistamines are frequently prescribed to patients with ABRS, there is minimal evidence supporting that they facilitate recovery. Although individuals may subjectively feel improvement after using these agents, objective rhinometric findings do not support this impression.11
Several randomized controlled trials assessed the efficacy of an additive effect of topical or oral decongestants or antihistamines to antimicrobials in adults with ABRS.111-113 Evaluation of the effect of topical decongestants (oxymetazoline) versus hypertonic (3%) saline, isotonic (0.9%) saline, or no topical treatment on mucociliary clearance illustrated a statistically significant improvement in mucociliary clearance only in the oxymetazoline and saline treatment groups.111 Even though significant improvement was observed in all treatment groups after 3 weeks, this was not significantly different from the group that received no topical treatment. Evaluation of the effect of topical oxymetazoline versus placebo did not find any significant difference in the treatment groups.113 An unexpected finding was that topical treatment with decongestants induced inflammation in the nasal cavity. This observation was confirmed in rabbits with experimental acute bacterial sinusitis.114
The efficacy of oral antihistamines (brompheniramine and phenylpropanolamine in syrup) in combination with nasal oxymetazoline versus placebo (oral syrup and nasal saline) was evaluated in the treatment of ABRS in children.115 All patients received 14 days of oral amoxicillin. The addition of a decongestant-antihistamine did not provide added benefit compared to placebo.
The adjunctive effect to standard treatment with antibiotics and oral steroids of the antihistamine H1 antagonist, loratadine, was examined in a double-blind placebo-controlled study in adults with acute exacerbation of allergic rhinosinusitis.116 All patients received amoxicillin-clavulanate for 14 days, plus oral prednisone. The degree of improvement was significantly greater for certain symptoms, including sneezing and nasal obstruction, in the loratadine group compared to control.
The recommendation against the use of decongestants or antihistamines as adjunctive therapy in ABRS places a relatively high value on avoiding the adverse effects from these agents and a relatively low value on the incremental improvement of symptoms, but these agents may still provide symptom relief in some patients with acute viral rhinosinusitis when antimicrobial therapy is not indicated.
Reduction in the viscosity and improvement in the quality of mucus can assist in resolution of the infection. Several methods achieve this goal, including nasal saline spray or irrigation, air humidification, adequate hydration, and mucolytic agents. Nasal saline irrigation or spray is a simple and effective method, available in the form of a nasal spray of sterile saline solution. It has been suggested that saline irrigation improves nasal symptoms by enhancing mucociliary function, mechanically clearing mucus, decreasing mucosal edema, and decreasing inflammatory mediators.117 Some discomfort is common during saline irrigation, and installation of nasal drops is less tolerated by babies.
The efficacy of intranasal saline irrigation in young children was evaluated in a clinical trial of 69 children with acute sinusitis (mean age 6 years, range 3-12).118 The children were randomized to receive either saline irrigations or no irrigation. The Total Nasal Symptom Scores as well as the Pediatric Rhinoconjunctivitis Quality of Life Questionnaire scores were significantly improved in the saline group. Furthermore, the nasal peak expiratory flow rate was significantly improved in the treatment group as compared to no irrigation.
Irrigation can be performed by dissolving half a teaspoonful of salt (about 3 g) in warm water (260 mL), with or without baking soda (about 0.5 g). The solution can be placed in a spray bottle or a syringe for nasal lavage. Sprays of saline (2 to 4 puffs at a time) are inhaled 3 times a day, and when necessary, the nasal secretions can be washed out with syringe rinsing and aspiration. Its use is recommended in both acute and chronic bacterial rhinosinusitis.
Inspired cool or hot humidified air and intake of adequate amounts of fluid are helpful in preventing and clearing thick secretions. Many mucolytic and mucoregulatory agents, as well as expectorants, are used to treat sinusitis. The most common is guaifenesin, which liquefies thick secretions effectively. It is available in liquid or tablet form, alone or in combination with oral decongestants.
Antihistamines are generally not used to treat bacterial sinusitis because they can thicken and dry the secretions, leading to crusting and further blocking of the osteomeatal complex. Antihistamines can be useful, however, if the underlying cause is allergic. Two classes of antihistamines are available. The first-generation antihistamines include diphenhydramine, hydroxyzine, promethazine, meclizine, chlorpheniramine, and tripelennamine. Newer, second-generation antihistamines cause less dryness and are nonsedating (ie, cetirizine, fexofenadine, and loratadine).
Intranasal budesonide with amoxicillin-clavulanate was found to enhance recovery as compared to pseudoephedrine in a randomized study that included 151 children with acute sinusitis.119 Intranasal corticosteroids (INCS) offer modest symptomatic improvement and minimal adverse events with short-term use. INCS are recommended as an adjunct to antibiotics in the empiric treatment of ABRS, primarily in patients with a history of allergic rhinitis. The beneficial effect of INCS could be due to their anti-inflammatory properties, which may reduce mucosal swelling and promote drainage. Steroids have a delayed onset of action, and clinical improvement may take 7 to 10 days. Corticosteroids are always used in conjunction with antimicrobial therapy.
The topical corticosteroids include fluticasone, budesonide, flunisolide, and triamcinolone acetonide, and they can be administered for prolonged periods, in contrast to topical decongestants. Topical agents can be delivered as an aerosol or as an aqueous solution. With prolonged use, topical side effects may occur (more often with the aerosol form than the aqueous form) and include irritation, sneezing, drying, a burning sensation, crusting, bleeding, and (rarely) septal perforation.109 Systemic corticosteroids are rarely necessary in the treatment of allergic rhinitis because of the generally good efficacy of topical corticosteroids. Immunotherapy may also be effective.120
Cromolyn sodium is available as a topical spray and helps to prevent perennial as well as seasonal allergic rhinitis.109 It works best when administered prior to exposure to an allergen and is given with a spray pump, in doses of 1 spray in each nostril every 4 hours during waking time. Relief is achieved between 4 and 7 days, whereupon the dose is reduced to an individual maintenance level. Side effects are infrequent, but they include irritation and sneezing. Cromolyn sodium is effective in the treatment of allergic rhinitis, but it does not help prevent the development of postviral sinus symptoms and nonallergic bacterial sinusitis.120 It is not recommended for use in sinusitis except to alleviate a concomitant allergic rhinitis.
Surgical drainage may be needed in cases that fail medical therapy, especially when complications occur. The goals of surgery are to allow drainage of purulent material and to prevent persistence, recurrence, progression, and complications. This is accomplished by removal of diseased tissue, preservation of normal tissue, promotion of drainage (or obliteration if this is not possible), and consideration of the cosmetic outcome. Functional endoscopic sinus surgery has become the main surgical technique used. Radical procedures are reserved primarily for acute or chronic rhinosinusitis complicated by orbital or intracranial involvement. Endoscopic surgery can achieve up to 76% to 96% success in both adults and children.121,122
Complications of sinusitis are uncommon, but they can be life-threatening when they occur. Diagnosis is assisted by finding local tenderness and dull pain, and it is confirmed by CT and nuclear isotope scanning.The most common causes of complications are anaerobic bacteria and S aureus. Management includes surgical drainage and antimicrobial therapy. Surgical debridement is rarely necessary after a properly extended course of parenteral antimicrobial therapy.123 Antibiotics should be administered for at least 6 weeks. Monitoring for possible intracranial complications is warranted.
Greater vigilance should be employed in treating patients with comorbidities, such as an impaired immune system, HIV, or cystic fibrosis.123 When not treated promptly and properly, sinus infection can spread via anastomosing veins or by direct extension to nearby structures.
Orbital complications were categorized by Chandler et al into 5 stages, according to their severity.124 Contiguous spread to the orbital area can result in periorbital cellulitis, subperiosteal abscess, orbital cellulitis, and abscess. Orbital cellulitis can complicate acute ethmoiditis if thrombophlebitis of the anterior and posterior ethmoidal veins spreads the infection to the lateral or orbital side of the ethmoid labyrinth.
Sinusitis can also extend to the central nervous system, where it can cause cavernous sinus thrombosis; retrograde meningitis; and epidural, subdural, and brain abscesses.123,124 Orbital symptoms often precede intracranial extension of the infection.125,126 Other emerging complications include sinobronchitis and maxillary and frontal bone osteomyelitis.123-127 Osteomyelitis of the frontal bone often originates from a spreading thrombophlebitis.128 A periostitis of the frontal sinus causes an osteitis and a periostitis of the outer membrane, which produces a tender, puffy swelling of the forehead. This requires closer follow-up, using broader-spectrum antibiotics, obtaining sinus cultures, and obtaining consultations from an otolaryngologist and an infectious diseases specialist. Antibiotics should be administered for at least 6 weeks. Monitoring for possible intracranial complications is warranted.
Even though consensus among experts has resolved many issues, many recommendations are based on expert opinion and are still challenged and open for further discussion. These issues include:
management strategies?
The majority of children with ABRS respond to empiric antimicrobial therapy, usually within a short period of time (3 days), and do not need to be admitted to the hospital. Those who continue to deteriorate despite broadened antibiotic coverage should be promptly referred to a specialist such as an otolaryngologist, allergist, immunologist, or an infectious disease specialist. Patients with a dentally associated sinusitis should be referred to a dentist for treatment of the underlying dental problem.
An otolaryngologist can confirm the diagnosis and assist in recovering the potential pathogens by collecting a specimen that can be analyzed microbiologically. This is especially important in immunocompromised individuals where an infectious diseases consultant can assist in the proper selection of antimicrobials and their dosing. Consultation with an allergist is helpful in those with recurrent infection and suspicion of immunologic disorder or allergy. Children with orbital or intracranial complications should be admitted to the hospital and need a prompt surgical and infectious disease consultation. Severe infection, particularly in immunocompromised patients or in patients with multiple medical problems that may complicate appropriate dosing or predispose to unusual microorganisms should be referred to an infectious disease specialist. Further work-up and consideration for hospitalization include suspicion of nosocomial sinusitis (recent intubation, feeding, or suction device), patients who are immunocompromised, or patients who may have meningitis or other intracranial complications or frontal or sphenoid sinusitis.
Rhinosinusitis is one of the most common complaints resulting in physician visits in the United States. An antecedent viral infection of the upper respiratory tract is the most common presentation. Despite its prevalence, the vast majority of cases resolve spontaneously, and only a small proportion develops a secondary bacterial infection that will benefit from antimicrobial therapy. Nonetheless, many individuals are treated with antimicrobials even though they do not suffer from bacterial sinusitis.
Because there are currently no good markers that define viral rhinosinusitis from bacterial, many emergency clinicians elect, when in doubt, to administer antimicrobials to their patients. This is often done because of nonmedical reasons, such as the desire to satisfy patients’ requests and the assumption that the use of antimicrobials will facilitate recovery and has no adverse effects. This common approach is one of the main contributors to the worldwide increase of resistance to antimicrobial agents of all respiratory tract bacterial pathogens that has made the management of true bacterial sinusitis more difficult. It is hoped that newer studies will explore the utility of adjuvant therapies as well as the correct timing of the introduction of antimicrobials to treat bacterial sinusitis. These studies can lead to development of better guidelines that may lead to the judicious use of these therapies.
The recent introduction of new vaccinations against S pneumoniae and the expected new vaccines against other potential sinus pathogens (ie, nontype screen b H influenzae ) may change the bacterial etiology. The increased recovery of MRSA is an example of such a change. Continuous monitoring of the evolving bacterial etiology of bacterial rhinosinusitis is, therefore, of great importance.
The family history of a sibling and parent who recently received antimicrobial therapy with a beta-lactam antibiotic placed her at greater risk of being infected with antimicrobial-resistant bacteria. This patient clearly demonstrated a case of acute bilateral maxillary sinusitis because she had purulent nasal secretion for over 10 days, signs of a cold, and inflammation that did not subside. No further diagnostic tests were required. Antimicrobial therapy was chosen empirically, with selection to provide coverage against all the potential pathogens that cause acute sinusitis (S pneumoniae, H influenzae, and M catarrhalis). She was placed on oral antimicrobial therapy with amoxicillin-clavulanate 500 mg q12h for 10 days in addition to antipyretics. The twice-a-day dosage of the agent and the low side-effect profile aided greater compliance. Her condition improved gradually, and she was symptom-free by 72 hours. Follow-up of 4 weeks later showed no recurrence of the sinusitis.


\











Evidence-based medicine requires a critical appraisal of the literature based upon study methodology and number of subjects. Not all references are equally robust. The findings of a large, prospective, randomized, and blinded trial should carry more weight than a case report.
The most informative references cited in this paper, as determined by the author, will be noted by an asterisk (*) next to the number of the reference.
Itzhak Brook
May 1, 2012