

Urgent care clinicians should be aware of the most current diagnostic and therapeutic recommendations for influenza and the resources available for guiding management. This review outlines the classification of these viruses, their pathophysiology, the identification of high-risk patients, and the importance of influenza vaccination. Seasonal variations of influenza are discussed, as well as the considerations regarding which patients to test based on the current local prevalence of disease. Given the significant overlap in clinical presentations, co-evaluation for COVID-19 is also briefly discussed in the context of the evaluation and management of influenza. Recommendations for use of the currently available antiviral treatments are discussed, as well as how to engage in shared decision-making with patients regarding risks and benefits of testing and treatment.
How would you manage these patients? Subscribe for evidence-based best practices and to discover the outcomes.
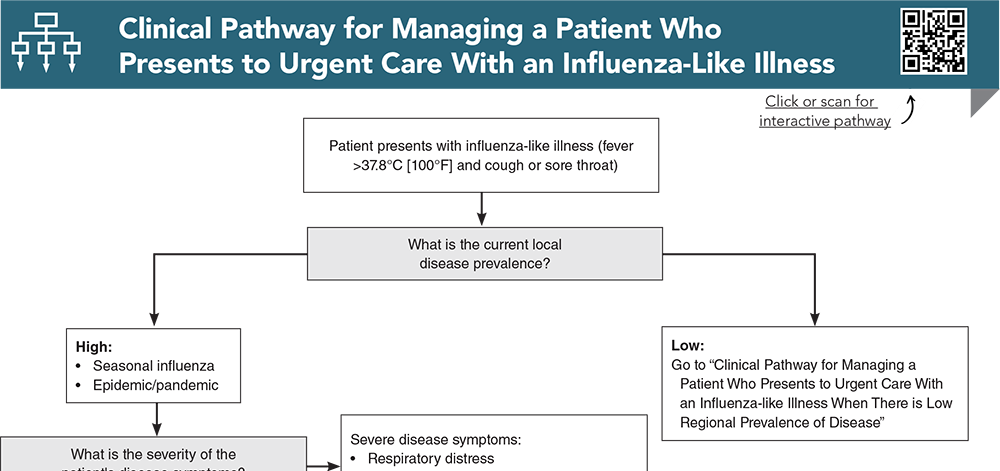
Subscribe to access the complete Clinical Pathway to guide your clinical decision making.
Buy this issue and
Following are the most informative references cited in this paper, as determined by the authors.
6. * Mossad SB. Another influenza season in the shadow of the COVID-19 pandemic. Cleve Clin J Med. 2021;88(11):594-597. (Commentary) DOI: 10.3949/ccjm.88a.21095
27. * World Health Organization. Recommended composition of influenza virus vaccines for use in the 2024-2025 northern hemisphere influenza season. Updated February 23, 2024. (WHO recommendations)
28. * Grohskopf LA. Ferdinands JM, Blanton LH, Broder KR, Loehr J. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2024-25 influenza season. MMWR Morb Mortal Wkly Rep. 2024;73(No. RR-5):1-25. (Recommendation)
34. * Greenhawt M, Turner PJ, Kelso JM. Administration of influenza vaccines to egg allergic recipients: a practice parameter update 2017. Ann Allergy Asthma Immunol. 2018;120(1):49-52. (Practice guideline) DOI: 10.1016/j.anai.2017.10.020
35. * Falsey AR, Treanor JJ, Tornieporth N, et al. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009;200(2):172-180. (Randomized controlled trial; 3837 patients) DOI: 10.1086/599790
38. * Immunization Action Coalition. Ask the Experts: Influenza. Updated September 5, 2024. Accessed November 11, 2024. (Website)
39. * Kroger A, Bahta L, Long S, Sanchez P. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Updated July 22, 2024. Accessed on November 11, 2024. (Practice recommendations)
40. * Toback S, Galiza E, Cosgrove C, et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir Med. 2022;10(2):167-179. (Randomized controlled trial; 400 patients) DOI: 10.1016/S2213-2600(21)00409-4
49. * Peltola V, Ziegler T, Ruuskanen O. Influenza A and B virus infections in children. Clin Infect Dis. 2003;36(3):299-305. (Retrospective study; 15,420 patients) DOI: 10.1086/345909
50. * Lim WS. Pandemic flu: clinical management of patients with an influenza-like illness during an influenza pandemic. Provisional guidelines from the British Infection Society, British Thoracic Society, and Health Protection Agency in collaboration with the Department of Health. Thorax. 2007;62(Suppl 1):1-46. (Clinical practice guidelines – UK) DOI: 10.1136/thx.2006.073080
51. * Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1-e47. (Clinical practice guideline) DOI: 10.1093/cid/ciy874
63. * Uyeki TM. Influenza diagnosis and treatment in children: a review of studies on clinically useful tests and antiviral treatment for influenza. Pediatr Infect Dis J. 2003;22(2):164-177. (Systematic review)
65. * Ozaras R, Cirpin R, Duran A, et al. Influenza and COVID-19 coinfection: report of six cases and review of the literature. J Med Virol. 2020;92(11):2657-2665. (Case reports and literature review; 6 patients) DOI: 10.1002/jmv.26125
83. * Jefferson T, Demicheli V, Rivetti D, et al. Antivirals for influenza in healthy adults: systematic review. Lancet. 2006;367(9507):303-313. (Systematic review; 51 randomized controlled trials) DOI: 10.1016/S0140-6736(06)67970-1
84. * Jefferson T, Demicheli V, Di Pietrantonj C, et al. Amantadine and rimantadine for influenza A in adults. Cochrane Database Syst Rev. 2006;2006(2):CD001169. (Cochrane review; 20 prophylaxis trials, 13 treatment trials) DOI: 10.1002/14651858.CD001169.pub3
Subscribe to get the full list of 92. references and see how the authors distilled all of the evidence into a concise, clinically relevant, practical resource.
Keywords: : influenza, flu, influenza-like illness, vaccine, H1N1, H3N2, H5N1, epidemic, pandemic, COVID-19, co-infection, vaccination, antiviral, oseltamivir, zanamivir, peramivir, baloxavir, neuraminidase inhibitors, rapid influenza test, RT-PCR, prevalence, resistance, chemoprophylaxis
Tracey Quail Davidoff, MD, FCUCM; Christopher Chao,MD
Lisa M. Campanella-Coppo, MD, FACEP
December 1, 2024
December 1, 2027 CME Information
4 AMA PRA Category 1 Credits™. 4 AOA Category 2-B Credits. 4 AAFP Prescribed Credits Specialty CME Credits: Included as part of the 4 credits, this CME activity is eligible for 2 Pharmacology CME credits and 4 Infectious Disease CME credits.