
ABC or Airway, Breathing, Circulation is a mantra of emergency medicine. There are numerous courses on airway management, most of which focus on assessment and intubation. However, post-intubation care (i.e., ventilator management) is often overlooked despite the critical importance of this component to patient outcome.
Understanding ventilators and their use is an essential competency in emergency medicine. First, appropriate ventilator management improves patient care while a mishandled ventilator is a dangerous tool. Indeed, misguided ventilator management can be a patient’s worst enemy and significantly worsen their prognosis. Second, emergency physicians are responsible for both the immediate and short-term care of intubated patients. In the current age of hospital overcrowding, intubated patients are spending an increasingly longer period of time in the ED; at times, they are even weaned and extubated in the ED before a bed becomes available upstairs.
You realize that it's been just a little too quite tonight when the radio suddenly cackles to life: "Teenage girl ... asthma … can't breathe … diaphoretic … giving nebs … No IV … 2-minute ETA." Within minutes, two medics rush in with a diaphoretic cyanotic girl perched forward on her hands. Her pleading glance catches yours as you watch her take her last voluntary breath; intubation is obviously required … ventilator management is your concern since you realize her life depends on it …. As you resuscitate the crashing asthmatic, your 60-year-old male patient on the other side of the curtain, who has been sleeping comfortably, begins to complain that his breathing is getting worse. He is a frequent flyer with a known history of bad emphysema and a worse attitude. He adamantly refuses ‘the mask' ventilation. You think back about his chest x-ray, which showed extensive bilateral pulmonary infiltrates, and wonder how long your luck can hold up before you need to intervene with him. His voice and attitude sound oddly weak, but you remember that the last time he was intubated he developed a pneumothorax ….
Just as you ponder these thoughts, a seasoned pair of medics burst into the ED. The hiss of nebs can be heard under the rushing sound of high pressure CPAP. "Sorry Doc, tried to raise you on the radio but no one answered. This lady is sick and not moving much air. We got her on CPAP at 20, 100%, but we're not making much progress; heart rate of 170 and can't get her sats higher than 60%. She's got CHF. Had no time to intubate." Just then their short, morbidly obese, pale, diaphoretic patient rips her CPAP mask aside and screams, "I … can't … breathe." Her eyes then roll back, and she begins to have a hypoxic seizure.
A broad-based search was employed using the Cochrane database of systematic reviews and PubMed. Key terms for the search included ventilators, ventilator management, mechanical ventilation, positive pressure ventilation, invasive positive pressure ventilation, noninvasive positive pressure ventilation, PEEP, auto-PEEP, emergency department, emergency room, respiratory failure, asthma, COPD, emphysema, CHF, congestive heart failure, and pulmonary edema. Articles were then categorized into type of study (e.g., prospective, randomized), consensus opinion, or review article. Priority was given to prospective, randomized, controlled studies. The references from these articles were also reviewed for additional sources of studies and reviews.
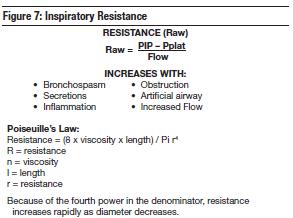
Asthma is an obstructive process associated with significant airway resistance secondary to bronchospasm, increased mucous production, edema, inflammation of the mucosal lining, and plugging of the bronchi and bronchioles. The energy required to overcome the frictional forces of respiration is primarily directed at overcoming airway flow resistance. This resistance can be quantified using Poiseuille's law which relates resistance directly to viscosity and inversely to the fourth power of the radius of the airway.
Approximately 20 million Americans have asthma.1 Asthma is the third leading cause of preventable hospitalization in the United States.2 It accounts for approximately 2 million visits to the ED each year.3,4 Direct health care costs in the United States were greater than $14.5 billion in the year 2000.5 This represents more than a 100% increase from the previous decade. About 10% of patients admitted to the hospital for asthma go to the intensive care unit; 2% of those are intubated.6 However, lower complication rates and fewer in-hospital deaths show that overall outcomes for severe asthma are improving.7
The loss of elastic recoil differentiates emphysema from asthma. Patients with emphysema experience both airway collapse and flow limitation during normal tidal breathing.45,65
Since elastic recoil of the lungs is key to outward flow of air, emphysematous patients have prolonged expiratory times and reduced outward flow. If the tidal volume is not completely exhaled, then a certain amount of air will be trapped in the alveoli. On x-ray, this hyperinflated lung appears lucent and is the characteristic picture for a COPD patient or the "pink puffer." The functional residual capacity (FRC) is the volume of gas remaining in the lungs at the end of normal exhalation; it is increased in this population.
The overall result of all these effects is increased dead space, severe ventilation-perfusion mismatch related to atelectasis, marked airflow limitation (bronchospasm, mucus, edema), air trapping (auto-PEEP), and hyperinflation (alveolar overdistention).66 Dynamic hyperinflation has the most deleterious effects, such as decreased cardiac output and increased risk of barotrauma.67-71Therefore, when managing a decompensated emphysematous patient on a ventilator, close attention must be paid to managing dynamic hyperinflation. This can be achieved by a combination of reduced respiratory rates (prolonged expiratory times), limited tidal volume, and shortened I-times.
On a ventilator, progressive hyperinflation results from either excessive tidal volume or insufficient expiratory time. Overinflated alveoli will increase dead space; this increases residual air within the lungs, reduces exchange of gases, and lowers alveolar ventilation. VT should be adjusted for each individual patient's lung mechanics rather than assuming that one VT will suit all patients. For patients with emphysema, a reasonable VT to begin with is 8 mL/kg PBW followed by a plateau pressure measurement. Plateau pressure is a reliable bedside indicator for alveolar overdistention. If plateau pressure is > 30 cm H2O, the VT should be reduced to 6 mL/kg PBW. Petrucci et al reported in a systematic review of 8 randomized controlled trials that tidal volumes adjusted up to 10 mL/kg PBW are acceptable if plateau pressure is < 30 cm H2O.72 As with an asthmatic on a ventilator, auscultation of the lungs during expiration is key to managing the ventilator and making adjustments to best match the patient's needs.
Acute respiratory failure in patients with emphysema is associated with significant expiratory obstruction and hyperinflation. Decreasing the amount of auto-PEEP on mechanical ventilation requires a decreased respiratory rate and prolonged expiratory phase. Since these patients often have less structural airflow obstruction than patients with asthma, initial rate settings can begin at 10-12 bpm. An end-expiratory occlusion maneuver should then be performed to measure auto-PEEP (air trapping). The exhalation phase can be expanded by decreasing the mechanical respiratory rate or shortening the I-time (Table 4).
An increase in respiratory rate proceeded by a decrease in expiratory time will worsen dynamic hyperinflation.
PEEP provides a fixed positive airway pressure at the end of expiration and opens previously unrecruited alveolar units. In the case of auto-PEEP due to flow limitation, increasing the PEEP setting on the ventilator should be set to counterbalance the auto-PEEP based on individualized lung mechanics.62-64,73 In emphysema, the different parts of the lungs are not affected to the same degree. Certain lung units may have longer time constants and therefore be more predisposed to hyperinflation than others.74 This increases the intricacy of determining ‘optimal' PEEP levels.
Unfortunately, there is insufficient data to support a generalized PEEP setting to prevent or minimize ventilator-associated lung injury while simultaneously preventing alveolar collapse. Evidence-based data is lacking to prove that high levels of PEEP are better.
Oxygen therapy provides benefits to patients who are hypoxemic. Oxygen should be administered in doses sufficient to raise the partial pressure of oxygen greater than 60 mm Hg8 or a pulse oximeter reading greater than 90%. The risk of oxygen-induced respiratory acidosis and hypercapnic respiratory failure associated with COPD is no longer a concern once the patient is intubated, sedated, and on controlled mechanical ventilation. However, prolonged use of high oxygen concentrations can lead to alveolar-capillary membrane changes and absorption atelectasis.75 Set the FIO2 at 100% upon the initiation of mechanical ventilation, and wean as early as possible to avoid extended uses as long as adequate serum partial pressure of oxygen is maintained.
The Starling relationship determines the fluid balance between the alveolar and vascular beds. In acute pulmonary edema, pulmonary capillaries enlarge and hydrostatic pressures overcome oncotic pressures. Fluid is ‘pushed' into the interstitial spaces and, in some cases, directly into the alveoli. This increased pulmonary fluid can be seen on chest x-ray with findings of Kerley B lines and a batwing perihilar radiographic picture. Gross effusions can develop as well. The fluid that enters the alveoli blocks ventilation of alveoli and disrupts the alveolar surfactant layer leading to alveolar collapse. Intrapulmonary shunting results following the alveolar consolidation (alveolar flooding) and alveolar collapse (atelectasis) (Figure 1). This will cause loss of functional alveoli and, consequently, reduced surface area for gas exchange.

As interventional cardiac care improves, more people are living with weaker hearts. Nearly 2% of the U.S. population has congestive heart failure. This percentage increases dramatically with age to include 5% in ages 60-69 and 10% over 70.13 Congestive heart failure (CHF) accounts for about 260,000 deaths per year.14 The number of ED visits for congestive heart failure in 2004 was 3.4 million.15 The annual cost for 2007 in the U.S. alone was $33.2 billion.16
When a person inspires, the diaphragm contracts and pulls the lungs downward. This produces negative pressure within the alveoli that is transmitted to the airway; air is then drawn in. During expiration, passive relaxation of the chest wall musculature allows the chest cavity to naturally recoil to elastic neutrality. Between each regular inhalation and exhalation, there is a brief expiratory pause when intrathoracic pressures match atmospheric pressures. This process is referred to as spontaneous negative pressure breathing. Ventilator breathing is markedly different in that ventilated lungs have air pushed rather than drawn into them. This is referred to as positive pressure ventilation. Positive pressure ventilation can be provided invasively (as when a patient is intubated and on full ventilatory support) or non-invasively (continuous positive airway pressure [CPAP] or bi-level positive airway pressure [BiPAP]).
Non-invasive positive pressure ventilation (NPPV) is the application of mechanical breaths without airway invasion and is delivered by a nasal or face mask. BiPAP delivers continuous positive airway pressure with different levels of inspiratory and expiratory support. NPPV has been a major advance in the management of acute respiratory distress. There are numerous randomized trials that demonstrate that NPPV lowers intubation rate, mortality, and hospital stay.17–24 Most recent studies suggest NPPV should be considered first-line therapy for patients with acute hypercapnic and hypoxemic insufficiency.25–29 A randomized controlled trial performed by Brochard et al compared 85 COPD patients and found the endotracheal intubation rate was 74% for controls receiving standard medical treatment compared to only 26% in the NPPV group.18 Despite the demonstrated advantages of NPPV, there are scenarios where the inappropriate application of NPPV may be harmful,30 and intubation is the only appropriate approach to managing the patient’s respiratory failure.
There are both good and poor candidates for NPPV (Table 1). Merlani et al published a retrospective study of 104 patients which found that NPPV failures had the following shared characteristics:31

Confalonieri et al analyzed prospective data collected from 1033 patients with COPD exacerbation and found the following variables to predict the risk of failure > 70% on admission:32
The risk increased to > 90% failure rate if a pH < 7.25 persisted after 2 hours on NPPV.
Evidence from prospective studies of over 500 patients demonstrates that early failed response to NPPV is highly predictive for invasive airway management and mechanical intervention.31–37
Ultimately, the decision to intubate is a clinical one. While data is important in directing decision making, clinical acumen or ‘gut instinct’ is probably the best guide in making this decision. There is no clinical study or evidence that suggests any single value for arterial pCO2, pH, or PaO2 by itself signifies an indication for mechanical ventilation. Clinical assessment of patient muscle fatigue, mental status, and stability relative to their entire emergent presentation should direct care.
The primary use of mechanical ventilation is to assist patients in generating sufficient alveolar ventilation
and oxygenation. Key to the successful management of the intubated patient is adequate sedation and analgesia; this is generally best accomplished through standing nursing protocols. Paralytics should be used sparingly, and other means should be employed to make the patient comfortable on the ventilator.
The most common abbreviations, acronyms, and terminology associated with mechanical ventilation are listed in Table 2.

A framework for remembering the basics of ventilator management is provided by the mnemonic:
MOVE AIR.
M = Mode
O = Oxygen
V = Volume (VT) or Pressure (PC)
E = Expiratory PEEP
A = Adequate Sedation
I = Inspiratory Time
R = Rate
While this mnemonic may be simplistic, attention to each of its components will help ensure quality and comprehensive care is offered.
The "mode" in mechanical ventilation describes how the ventilator controls pressure, volume, and flow for each breath, along with a description of how the "breaths" are sequenced.39 No evidence conclusively proves one mode of ventilation provides a better outcome than another for initial management of acute respiratory failure. The principles of ventilation are more important than the mode used to practice them. The most commonly used mode is assist/control. Assist/control covers both aspects of volume control (AC) and pressure control ventilation (PC/AC).
During AC, the ventilator will deliver a set number of breaths at a preset tidal volume (in volume control mode) or pressure control level (in pressure control mode, adjustments in pressure are necessary until the desired expired volume is achieved). In the absence of a patient's spontaneous effort, the ventilator will deliver the ‘controlled' breaths. This approach ensures a minimum level of minute ventilation. A set respiratory rate is compulsory for an adequately sedated and possibly paralyzed patient since the ventilator does all the work.
Volume-controlled breaths deliver a constant volume with constant flow.
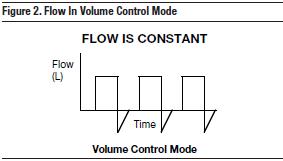
Flow = Tidal Volume/Inspiratory Time
Since flow is constant, inspiratory flow can be set and adjusted by manipulating the inspiratory time (I-time), flow knob, or % I-time knob based on the total respiratory rate. When using a volume mode, a constant volume will be delivered, but pay close attention to the peak pressure alarm to prevent barotrauma. While the volume delivered remains constant, airway pressure will vary dynamically based on lung compliance and airway resistance. If set correctly, the peak pressure alarm will indicate changes in any of these variables. Peak pressure alarm should be set < 10 cm H2O above the PIP to deliver an acceptable tidal volume.
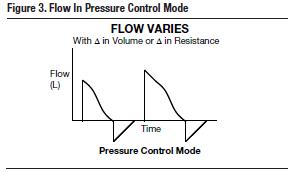
A pressure-controlled breath provides a constant preset pressure. In this mode, the tidal volume (VT), rather than the airway pressure, is variable. Flow is variable as well, as the ventilator delivers as much as is needed to achieve the preset pressure limit. Therefore, volume and flow will change based on the patient's lung compliance and airway resistance. Pressure is set with a target volume in mind. The volume is only a "target" because, unlike volume control, the VT may be higher or lower than intended. On the alarm settings, the tidal volume parameters are set above and below this ‘target' volume so the alarm will sound if the patient's volumes are outside of the desired range. As opposed to volume control mode, where the ventilator is set to alarm for high pressures, in pressure control mode, setting a low and high VT limit will serve as an alert to inappropriate changes in volume delivered as a result of dynamic alteration in the patient's lung mechanics.
As an aside, synchronized intermittent mandatory ventilation (SIMV) is a form of assist control where the ventilator is programmed to deliver a set number of breaths at either a predetermined tidal volume or pressure control level. SIMV allows the patient to PEEPRATEbreathe spontaneously between mandatory breaths from the ventilator. In this mode, patients retain some of the work of breathing as they add pressure support breaths between the set ventilator rate.

Oxygen saturation should be maintained > 90%. The concentration of oxygen delivered is adjusted by changing the FIO2 (fractional percentage of oxygen) from 21% (room air) to 100%. An arterial blood gas (ABG) will calculate the initial partial pressure of oxygen (PaO2) and the arterial partial pressure of carbon dioxide (PaCO2). These values can be correlated to noninvasive end tidal CO2 and pulse oximetry to allow for a noninvasive means of guiding ventilator adjustment. It is reasonable to adjust FIO2 according to the pulse oximeter as long as a good waveform is available and corresponding correctly with the rhythm strip and ABG. A reasonable goal is to reduce the delivered oxygen concentration to < 50%.40
Volume or, more specifically, tidal volume (VT) is the amount of gas the ventilator delivers during eachmachine breathing cycle. A couple of caveats must be kept in mind. First, volume is certainly dependent on the mode used. A volume mode delivers a preset volume whereas a pressure mode delivers a variable volume based on the preset pressure and the flow resistance of the airway. Additionally, a set volume does not always equal the delivered tidal volume as air is lost along leaks and in mechanical dead space. Mechanical dead space, or compressible volume, represents volume lost in distention of the ventilatory circuit. Adjusting the tidal volume setting on the ventilator will help resolve volume loss.
PEEP (also known as extrinsic PEEP) is the airway pressure maintained by the ventilator during the expiratory phase. Ventilator-added PEEP helps to augment the baseline expiratory pressure, to ‘splint open' the airways during expiration, and to decrease physiologic alveolar shunting. This maximizes gas exchange while minimizing overdistention of the lungs. PEEP helps maintain functional residual capacity and ‘works' at the alveolar level.
Passive ventilation occurs when the patient is sedated and, if necessary, paralyzed. In this scenario, all respirations are machine triggered and the patient does not initiate any spontaneous breaths. This allows for control over a patient's alveolar ventilation through manipulation of minute ventilation, respiratory rate, and I:E ratio. This approach not only rests the patient but also helps to avoid dynamic hyperinflation and ventilator-induced lung injury (VILI) often associated with obstructed expiratory flows, collapsed airways, and/or patient-ventilator dyssynchrony.
Adequate sedation is also necessary to determine appropriate settings for mechanical ventilation based on lung mechanics such as airway resistance, auto-PEEP (air trapping) and plateau pressures (alveolar overdistention). Measurement of these values is only accurate when the patient is properly sedated.41 For the first 12-24 hours of mechanical ventilation, sedation is normally required to optimize ventilator settings, to detect adverse effects from mechanical ventilation, and to minimize complications due to mechanical ventilation.
Inspiratory time is the period between inspiration and the start of expiratory flow. I-time is a mandatory setting and will remain constant from breath to breath. In volume control mode, the I-time can be adjusted by 3 different settings: desired I-time, % I-time (% based on set respiratory rate), or the flow rate knob. In contrast, for pressure control, I-time can only be adjusted by 2 settings: desired I-time or % I-time knob. The patient's expiratory time is calculated based on the set inspiratory time and respiratory rate (Table 4). The expiratory time represents the time during which a patient passively exhales. Since I-time is constant, E-time will vary if the patient is adding breaths to the set rate. Therefore, in order to control the I:E ratio, the patient must be adequately sedated.
During the initial management of a fully sedated ventilated patient, the respiratory rate is determined by the ventilator setting (Table 3). Minute ventilation is equal to the product of tidal volume and respiratory rate. The set respiratory rate must take into consideration the patient's ability to exhale in order to avoid lung overdistention. The typical starting respiratory rate for a healthy adult is 8-14 bpm.

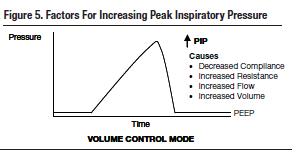
Valuable information can be obtained for optimal settings of mechanical ventilation based on your patient's respiratory mechanics (Figures 6 and 7). The assessment of lung mechanics is essential for the effective management of ventilated patients. Failure to obtain the right data can be dangerous for your patient. Common dynamic changes and their associated sequelae are: alveolar overdistention that can result in VILI, increased flow resistance occurring with bronchospasm in asthma, decreased lung compliance in acute pulmonary edema, and dynamic hyperinflation in emphysema.
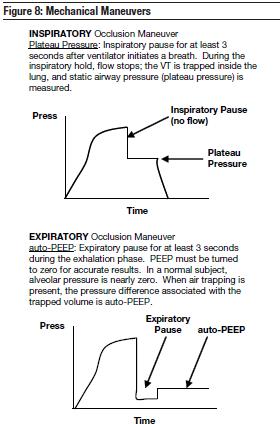
One of the first parameters to measure is auto-PEEP. This will determine the appropriate PEEP level42 and uncover any air trapping.43 To do this, auto-PEEP must be determined; this is accomplished by first setting the PEEP to zero to measure the actual auto-PEEP with an exhalation hold maneuver (Figure 8). The intrinsic (auto-) PEEP may not be accurately detected if the extrinsic PEEP is set greater than the auto-PEEP. Sedation is key because expiratory muscle contraction and chest and abdominal wall contributions to ventilation can alter the measurements of auto-PEEP and produce falsely elevated levels of auto-PEEP.44,45 This can worsen dynamic hyperinflation and overdistention by leading to a mistakenly higher level of applied PEEP.
Alveolar overdistention is a precursor to the development of acute lung injury (ALI). Plateau pressure is the pressure equilibration between the airways and alveoli. Plateau pressure is a measurement of alveolar overdistention; its clinical value can provide helpful information in determining appropriate tidal volumes for each patient. Plateau pressure is achieved by pausing flow at end-inspiration by initiating an inspiratory hold (Figure 8). Patients must be heavily sedated or paralyzed to obtain reliable measurements.
Airway resistance is not simply a flow inward problem. Asthmatics must struggle both to inhale and to exhale. Longer expiratory times are necessary for the entire volume of gas to be released. Air trapping occurs when an asthmatic either cannot fully exhale before the next breath or cannot overcome the frictional resistance in their own airways to empty their alveoli. Either mechanism leads to retained air in the alveoli and increased alveolar pressures. This is referred to as air trapping. This additional pressure at the end of expiration is referred to as intrinsic PEEP or auto-PEEP.
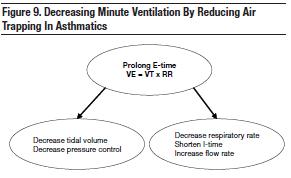
Mechanical ventilation can lead to or worsen gas trapping if insufficient expiratory times are provided. In this situation, the lungs become hyperinflated (dynamic hyperinflation) and overdistended. Excessive dynamic hyperinflation can lead to hypotension and volutrauma during mechanical ventilation of severe asthma as demonstrated in the comparative study of 95 patients by Williams et al.46 Since this increases the chances of morbidity and mortality,47 strategies must be employed to avoid this complication. The most effective way to reduce the worsening effects of lung hyperinflation is through a reduction in minute ventilation (VT x RR) (Figure 9).48-50
The deliberate use of alveolar hypoventilation to reduce pulmonary overdistention and acceptance of hypercapnia has been termed "permissive hypercapnic ventilation."48,51 Well-designed studies (such as a prospective randomized controlled trial of 53 patients with ARDS performed by Amato et al52 and a retrospective controlled study of 34 cases involving status asthmaticus by Darioli et al53) have shown the resulting acidosis and increased carbon dioxide in the setting of controlled hypoventilation to be generally well tolerated. In a systematic review of over 20 clinical studies, Bigatello et al found that although hypoventilation is a reasonable alternative to invasive ways of limiting VILI, there is no clear evidence that demonstrates a safe level of hypercapnia and acidemia.54 The reduction in minute ventilation has the potential for lowering serum oxygenation. The use of supplemental oxygen during permissive hypercapnia ventilation allows for the substantial reduction of minute ventilation without jeopardizing oxygenation.
Fundamental to permissive hypercapnia is sedation and possibly paralysis. The prolonged use of neuromuscular-blocking agents in combination with corticosteroid therapy increases the risk of the development of protracted muscle weakness.55 In a retrospective cohort study, Leatherman et al evaluated 107 consecutive episodes of mechanical ventilation for severe asthma to assess the incidence of myopathy in patients treated with corticosteroids alone versus corticosteroids with neuromuscular paralysis.56 The use of corticosteroids and a neuromuscular-blocking agent was associated with a higher incidence of muscle weakness as compared with the use of corticosteroids alone (20 of 69 versus 0 of 38). Eighteen of the 20 weak patients had been paralyzed for more than 24 hours. However, this is not a concern in the initial management of the acute asthmatic in the ED. Having said that, paralytic agents should be used only if adequate control of mechanical ventilation cannot be achieved by sedation alone. Minimizing the use of paralytic agents may decrease the risk of critical illness myopathy and other adverse consequences of muscle paralysis.57
Since the most effective method of decreasing dynamic hyperinflation is by reducing the minute ventilation (VE = RR x VT), patients with asthma will benefit from reduced tidal volumes. Protective lung ventilation starts at 6 mL/kg predicted body weight (PBW) and increases to 8-10 mL/kg PBW as long as the plateau pressure remains < 30 cm H2O.58 Similarly, if the plateau pressure is above 30 cm H2O at a VT of 6 mL/kg PBW, then VT should be decreased to 4-5 mL/kg to keep the plateau pressure below 30 cm H2O. This strategy will minimize the potential of developing VILI injury.
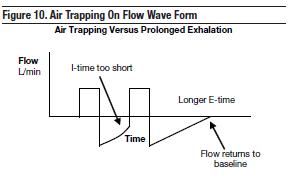
Reduced respiratory rates and shortened I-times will allow longer exhalation times, reduce air trapping, and help minimize VILI caused by alveolar overdistention.48 Adjusting inspiratory time versus reducing respiratory rate is summarized in Table 4. Prolongation of expiratory time has been shown to decrease dynamic hyperinflation in patients with severe asthma; this was demonstrated in an observational study by Leatherman et al of 12 mechanically ventilated patients.59 The reduction in respiratory rate allowed for longer E-times, and the decrease in dynamic hyperinflation was evidenced by a reduction in plateau pressure. This is accomplished by beginning with a rate between 6 bpm and 8 bpm with the main goal of ensuring low minute ventilation (VE) and an expiratory time long enough to allow lung emptying; lung emptying is observed on the flow verses time waveform on the ventilator. The expiratory flow waveform optimally should return to baseline before the next breath begins (Figure 10).
Finally, auscultation of the intubated asthmatic is critically important. While the ventilator can offer significant information, listening for complete expiration (end of wheezing) before the next breath begins is paramount to preventing air trapping and dynamic hyperinflation.
The presence of air flow at end-expiration indicates that the alveolar pressure is higher than the applied PEEP.44 The challenge at the bedside is to select the appropriate amount of PEEP to counterbalance auto-PEEP (also called intrinsic PEEP). To do this, auto-PEEP must be determined by the expiratory occlusion maneuver (Figure 8). PEEP can have a significant positive effect in the care of an asthmatic and should not be overlooked. Well-designed prospective studies of over 60 patients have shown that the addition of PEEP in the setting of auto-PEEP will prevent gas-trapping by holding the airways open.60-62 The appropriate use of PEEP will provide alveolar stability and reduce potential injury that occurs during recruitment and decruitment of atelectatic alveoli.63 However, PEEP levels of 10 cm H2O or higher can increase lung volume in patients with asthma and lead to lung overdistention.64 The use of PEEP during total ventilatory support of a patient who has dynamic hyperinflation with airflow obstruction due to acute asthma and without airway collapse is controversial.
The loss of elastic recoil differentiates emphysema from asthma. Patients with emphysema experience both airway collapse and flow limitation during normal tidal breathing.45,65
Since elastic recoil of the lungs is key to outward flow of air, emphysematous patients have prolonged expiratory times and reduced outward flow. If the tidal volume is not completely exhaled, then a certain amount of air will be trapped in the alveoli. On x-ray, this hyperinflated lung appears lucent and is the characteristic picture for a COPD patient or the "pink puffer." The functional residual capacity (FRC) is the volume of gas remaining in the lungs at the end of normal exhalation; it is increased in this population.
The overall result of all these effects is increased dead space, severe ventilation-perfusion mismatch related to atelectasis, marked airflow limitation (bronchospasm, mucus, edema), air trapping (auto-PEEP), and hyperinflation (alveolar overdistention).66 Dynamic hyperinflation has the most deleterious effects, such as decreased cardiac output and increased risk of barotrauma.67-71 Therefore, when managing a decompensated emphysematous patient on a ventilator, close attention must be paid to managing dynamic hyperinflation. This can be achieved by a combination of reduced respiratory rates (prolonged expiratory times), limited tidal volume, and shortened I-times.
On a ventilator, progressive hyperinflation results from either excessive tidal volume or insufficient expiratory time. Overinflated alveoli will increase dead space; this increases residual air within the lungs, reduces exchange of gases, and lowers alveolar ventilation. VT should be adjusted for each individual patient's lung mechanics rather than assuming that one VT will suit all patients. For patients with emphysema, a reasonable VT to begin with is 8 mL/kg PBW followed by a plateau pressure measurement. Plateau pressure is a reliable bedside indicator for alveolar overdistention. If plateau pressure is > 30 cm H2O, the VT should be reduced to 6 mL/kg PBW. Petrucci et al reported in a systematic review of 8 randomized controlled trials that tidal volumes adjusted up to 10 mL/kg PBW are acceptable if plateau pressure is < 30 cm H2O.72 As with an asthmatic on a ventilator, auscultation of the lungs during expiration is key to managing the ventilator and making adjustments to best match the patient's needs.
Acute respiratory failure in patients with emphysema is associated with significant expiratory obstruction and hyperinflation. Decreasing the amount of auto-PEEP on mechanical ventilation requires a decreased respiratory rate and prolonged expiratory phase. Since these patients often have less structural airflow obstruction than patients with asthma, initial rate settings can begin at 10-12 bpm. An end-expiratory occlusion maneuver should then be performed to measure auto-PEEP (air trapping). The exhalation phase can be expanded by decreasing the mechanical respiratory rate or shortening the I-time (Table 4).
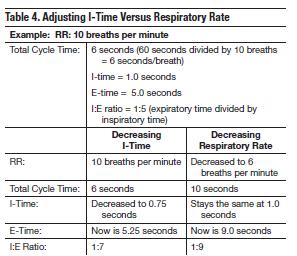
An increase in respiratory rate proceeded by a decrease in expiratory time will worsen dynamic hyperinflation.
PEEP provides a fixed positive airway pressure at the end of expiration and opens previously unrecruited alveolar units. In the case of auto-PEEP due to flow limitation, increasing the PEEP setting on the ventilator should be set to counterbalance the auto-PEEP based on individualized lung mechanics.62-64,73 In emphysema, the different parts of the lungs are not affected to the same degree. Certain lung units may have longer time constants and therefore be more predisposed to hyperinflation than others.74 This increases the intricacy of determining ‘optimal' PEEP levels. Unfortunately, there is insufficient data to support a generalized PEEP setting to prevent or minimize ventilator-associated lung injury while simultaneously preventing alveolar collapse. Evidence-based data is lacking to prove that high levels of PEEP are better.
Oxygen therapy provides benefits to patients who are hypoxemic. Oxygen should be administered in doses sufficient to raise the partial pressure of oxygen greater than 60 mm Hg8 or a pulse oximeter reading greater than 90%. The risk of oxygen-induced respiratory acidosis and hypercapnic respiratory failure associated with COPD is no longer a concern once the patient is intubated, sedated, and on controlled mechanical ventilation. However, prolonged use of high oxygen concentrations can lead to alveolar-capillary membrane changes and absorption atelectasis.75 Set the FIO2 at 100% upon the initiation of mechanical ventilation, and wean as early as possible to avoid extended uses as long as adequate serum partial pressure of oxygen is maintained.
Pulmonary edema is one of the most common causes of absolute capillary shunting. The overall result is reduced serum oxygen tension and relative hypercarbia. Patients presenting with capillary shunting respond poorly to oxygen therapy alone because the inspired oxygen cannot adequately reach the circulating blood. Positive pressure ventilation, either noninvasive or invasive, can help reverse the decompensated physiologic state and push fluid back out of the lungs.63,76
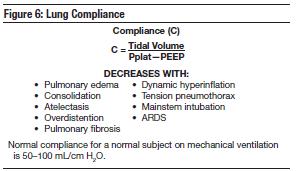
In congestive heart failure, lung compliance is markedly reduced secondary to engorged capillaries surrounding the alveoli, alveolar flooding, and alveolar collapse. Low compliance means that volume change is small per unit pressure change (C = Δ Vol/Δ Press). The decrease in lung compliance restricts the expansion of the lungs and physiologically functions as a restrictive defect. As such, patients with acute pulmonary edema will benefit from a reduction in their tidal volumes.
Normal VT in a less compliant lung may cause alveolar overdistention and lead to an increase in plateau pressure. An initial 6 mL/kg PBW should be a starting point to reduce the risk of iatrogenic acute lung injury. Since plateau pressure is an indicator of alveolar overdistention, the VT can be adjusted up or down based on the patient's lung mechanics. If plateau pressure can be maintained <30 cm H2O, the likelihood of ventilator-induced lung injury is minimal.60
Since smaller tidal volumes are employed, higher respiratory rates must be used to maintain a sufficient minute ventilation (VE = VT x RR). Remember: the goal for plateau pressure is < 30 cm H2O. Patients with a restrictive lung disease, such as pulmonary edema, tolerate a respiratory range between 12 bpm and 20 bpm since they typically do not experience the obstructive components associated with air trapping.
The PaO2/FIO2 can be used as an index of pulmonary oxygen exchange efficiency (Table 5). A low PaO2/FIO2 ratio on a high FIO2 > .60 is evidence of a poor response to oxygen therapy and of absolute shunting. A high FIO2 is ineffective in improving PaO2 in the presence of a large intrapulmonary shunt because oxygen cannot reach the blocked alveoli. This is referred to as refractory hypoxemia. High FIO2 levels predispose patients to the development of absorption atelectasis77and exacerbate the capillary shunting process. Decreases in lung volume, alveolar collapse, and hypoxia stimulate an increased pulmonary vasomotor tone by the process of hypoxic pulmonary vasoconstriction.77Therefore, the appropriate use of oxygen to relieve hypoxic pulmonary vasoconstriction and right heart strain will lessen myocardial ischemia, improve cardiac output, and improve oxygen delivery to other critical organs.

The role of PEEP cannot be stressed enough in acute pulmonary edema. An optimal level of PEEP will help splint open water-logged airways as well as reverse continued hydrostatic fluid flow into alveoli. PEEP will recruit and prevent alveolar collapse, increase lung compliance, and reduce the intrapulmonary shunt.63,78
PEEP improves functional residual capacity. For every change in PEEP, compliance and plateau pressure should be assessed to avoid alveolar overdistention and the risk of barotrauma. Since there is no data that supports a minimal level of PEEP for this population, start with a PEEP between 8 cm H2O and 10 cm H2O and adjust accordingly to keep both plateau pressures < 30 cm H2O and PaO2 between 60 mm Hg and 80 mm Hg.
PEEP affects cardiac function from increased lung volumes and intrathoracic pressure.78,79 An optimal level of PEEP improves the hemodynamics of congestive heart failure by 5 primary components:80,81
In patients with cardiac dysfunction, Fellahi et al reported in a randomized controlled study of 12 patients that PEEP of 9.6 +/- 0.2 cm H2O could improve rather than impair cardiac output by decreasing left ventricle afterload in a manner similar to systemic vasodilator therapy; however, it cannot be recommended in patients with dilated cardiomyopathy and poor left ventricular function.82 The analogous manner to treat CHF with PEEP, as with vasodilators, was also supported in a well-designed randomized study of 39 patients. This study demonstrated a rise in cardiac output when PEEP was appropriately applied to patients with poor myocardial function.83 Cardiac output showed curvilinear changes with an increase in PEEP from 0 cm H2O to 7 cm H2O, but it dropped markedly when PEEP was increased to 13 cm H2O.
If the ventilator is not used properly, the patient can be injured. Physicians must be keenly aware of not only the post-intubation period and appropriate ventilator set up, but also of the dynamic nature of the disease they are managing. VILI describes a compilation of complications associated with the use of a ventilator.
Ventilator strategies that minimize air trapping, dynamic hyperinflation, and auto-PEEP can be achieved by decreasing the set mechanical respiratory rate, prolonging the expiratory phase of ventilation, reducing VT, and shortening I-time.48,64,69,84 Elimination of patient effort through sedation and, if necessary, paralysis is employed to avoid patient-ventilator dyssynchrony. These strategies will reduce the risks for barotrauma and VILI caused by auto-PEEP and alveolar overdistention; see "Ten Pitfalls To Avoid."
Normal physiologic VT is typically around 6-8 mL/kg PBW throughout life. Until recently, most patients with acute respiratory failure were managed with VT of 10-15 mL/kg PBW. VILI can occur when the lungs and alveoli overdistend.85 It is quite clear in the evidence of 2 randomized, controlled trials of over 900 patients60,86that VT ≥ 12 mL/kg PBW in acutely ill patients is associated with increased mortality and that smaller tidal volumes of 6-8 mL/kg PBW were effective in the management of acute lung injury as they avoided excessive alveolar distention. Along similar lines, patients with obstructive and restrictive processes who are at risk for VILI may also benefit from lung-protective ventilation.87,88 However, there is insufficient data to recommend a VT of 6 mL/kg PBW as a standard for every patient who requires mechanical ventilation.
Plateau pressures are determined by static measurement of pulmonary distention pressures. In the prospective, randomized, controlled trial reported by the ARDS Network, 861 patients were placed on either low VT (6 mL/kg PBW) with plateau pressures < 30 cm H2O or on higher VTs (12 mL/kg PBW) with plateau pressures up to 50 cm H2O.60 The results showed a significant decrease in mortality from 39.8% to 31% (P = 0.007) in patients receiving lower tidal volumes and plateau pressures. If VT is limited, fewer patients reach higher plateau pressures so that alveolar overdistention and excessive stretch is minimized. Since plateau pressure is a better indicator of transpulmonary pressure and alveolar overdistention, it is a better target for determining appropriate VT settings (Figure 8).
Comparative systematic reviews of lung-protective ventilation studies suggest that plateau pressures rather than tidal volumes are the most accurate bedside indicator of alveolar overdistention for patients with ARDS and those at risk for VILI.74,89
Patients who require smaller tidal volumes to minimize plateau pressures and reduce lung injury have other potential adverse effects including hypercarbia and respiratory acidosis. However, respiratory acidosis without a co-existing severe metabolic acidosis is generally safe and well tolerated.90
High breathing rates shorten the time available for expiration. Expiratory resistance develops when airways are narrowed by bronchospasm, mucus hypersecretion and plugging, mucosal or interstitial edema, airway inflammation, airway collapse, or loss of elastic recoil of the lung. Patients with severe airflow obstruction and impaired expiratory flows may not have enough expiratory time to exhale all of the air from the lungs. If expiratory time is not long enough, air is trapped in the alveoli. This is known as air trapping. When this continues over several breaths, ‘breath stacking' will occur until a new end-expiratory lung volume is achieved. As this volume increases, the functional residual capacity (FRC) of the lung increases, forcing normal tidal breathing to occur at higher lung volumes. The lungs become overdistended and hyperinflated. This is referred to as dynamic pulmonary hyperinflation. Pulmonary hyperinflation can worsen gas exchange by increasing physiologic dead space, reducing oxygen delivery to the tissues, and increasing the risk of barotrauma (Figure 11).48
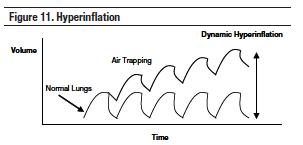
The presence of airflow at end-expiration indicates that the alveolar pressure is higher than the atmospheric pressure or higher than the applied PEEP.44,45 The pressure difference associated with the trapped volume is called intrinsic PEEP or auto-PEEP. Auto-PEEP is the average pressure inside the alveoli at end-exhalation. Elevated intrathoracic pressure due to auto-PEEP can decrease cardiac output and blood pressure by impeding venous return and right ventricular filling. This will result in increasing pulmonary vascular resistance and increasing preload and afterload of the left ventricle (Figure 8).91
Managing a ventilated patient involves obtaining the correct information and maximizing your ventilator settings. Having the right PEEP setting is very important. PEEP serves to splint open small airways and to recruit alveoli. Determining a patient's optimal PEEP is done through the clinical procedure known as the P-flex.
To understand P-flex we need to recall some basic physics. To move a body at rest requires overcoming friction (a type of resistance). There is static and dynamic resistance; the difference is very important. The initial movement of an object (static friction) requires significant energy to overcome the static forces holding the object in place. Once the object begins to move, less but continuous force must be exerted to continue the objects movement (overcoming dynamic friction).
Inside the lungs of an ill patient, the alveoli tend to collapse secondary to reduced patient effort to keep them open. One goal of ventilator management is to ‘pop' (static friction) alveoli open and to use them for ventilation (alveolar recruitment). This can be facilitated by optimizing PEEP. The P-flex helps select the appropriate PEEP setting to open the collapsed alveoli. On a ventilator, pressure volume curves can be created. Compliance represents the proportion of change in volume to that of change in pressure. (C = Δ Vol/Δ Press). The pressure volume-curve (P-V curve) plots the two components (inspiration and expiration) throughout the respiratory cycle. With regards to alveolar recruitment, inspiration is key.
Applying this to collapsed alveoli and static/dynamic resistance is demonstrated in Figure 12. On the left side of the plots (just below the lower inflection point [LIP]), compliance is low and a significant amount of pressure must be exerted to obtain a relatively small change in volume. However, the forces required to change volume (i.e., high compliance) change dramatically at the lower inflection point up to the upper inflection point (UIP). Between these points, a relatively small change in pressure results in significant volume change. The inspiration between LIP and UIP represents optimal lung usage. In clinical terms, the goal is to keep patients inspiring between the LIP and UIP. Setting the appropriate PEEP will allow the start of inspiratory cycle at the LIP. Determining this number is done through a P-flex calculation.
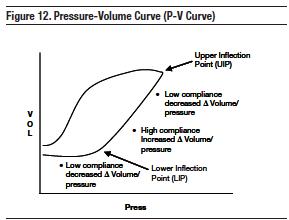
Gattinoni et al introduced "P-flex" as the intersection point between the slopes of the low compliance segment and high compliance segments (Figure 13).92 The intersection of these 2 slopes was defined as the P-flex and corresponds to the optimal PEEP for the patient. Alveolar recruitment is maximized at this point.
By using the P-flex, PEEP can be optimized. This will help reduce the need for high inspiratory pressures (which were being used to overcome the static resistance of the alveoli) and help avoid barotrauma to the patient. In simple terms, the P-flex helps us to determine the appropriate pressure to keep our alveoli moving (open) and to avoid repeatedly overcoming the static resistance of collapse and re-recruited alveoli.
In a randomized controlled study of 52 patients by Amato et al,88 setting PEEP 2 cm H2O above the LIP had less barotrauma and a lower mortality rate when compared to conventional strategies without P-V curve analysis. The rationale for setting PEEP 2 cm H2O above the LIP was to completely avoid any chance of recruitment and decruitment of alveoli that would cause lung injury.
Unlike the expiratory-hold occlusion maneuver, the advantage of a P-V curve is that auto-PEEP can be measured without reducing the PEEP to 0, thus minimizing the shear forces of opening and closing alveoli. However, adequate sedation and/or paralysis is still required since the patient's flow rate must be < 10 L/min to eliminate resistive changes in the lung.93,94 PEEP titration by P-flex and P-V curve analysis is still an ongoing investigational process simply due to the range in variability of subjective interpretation.95–97 Further studies in the overall use of its clinical capabilities when combined with other techniques will be a helpful tool in decreasing the risks of barotrauma and VILI.
Pressure-regulated volume control is a mode that allows the physician to use pressure control ventilation with a constant tidal volume. One way to look at PRVC is that it combines assist control volume mode and pressure control mode. This approach helps us to maintain adequate ventilation (tidal volume) while avoiding excessive alveolar pressures.
The tidal volume is the key setting, but the ventilator delivers a pressure-controlled breath. The pressure to deliver the breath will automatically adjust to changes in a patient's compliance and airway resistance until the set tidal volume is achieved. If the delivered volume is insufficient on one breath, the ventilator increases inspiratory pressure for the next breath. Additionally, the ventilator will decrease the pressure delivered if tidal volume is too high.
The maximum amount of pressure the ventilator is allowed to deliver in PRVC is determined by the high-pressure alarm setting. The peak pressure allowed is 5 cm H2O below this setting. For example, the high-pressure alarm should be set at 35 cm H2O to assure that the patient will not exceed a PIP > 30 cm H2O. Since pressure-controlled breaths equal plateau pressure, recall that it is recommended to keep plateau pressures < 30 cm H2O to avoid lung overdistention.
The advantage of PRVC is that minute ventilation and tidal volume are guaranteed with controlled peak airway pressures, thus reducing the risk for VILI.
Mandatory settings for PRVC are:
Ventilators are life-saving machines, but they must be used properly. Just as they can save lives, they can cause significant and irreparable damage to a patient if used improperly. In this article, the basics of ventilator management were discussed for 3 common causes of respiratory failure in the ED. While respiratory decompensation was the common theme, each disease process is marked by unique pathophysiology. An understanding of the diseases, their pathophysiology, and the ventilator basics presented here will allow for confident management of difficult patients.
Asthma is a major health concern in the United States. This disease affects both young and old, and it can rapidly lead to respiratory insufficiency and arrest. The underlying issue involves obstruction of the lower airway with inflammation, mucous, and airway spasm. Ventilatory management must be approached carefully and individualized to each case. Adequate sedation is the first step. This will allow a tired patient to rest and allow accurate lung mechanics measurements to be obtained. Ensuring adequate expiratory times as well as avoiding excessive airway pressures (plateau not inspiratory pressures) is key. Auscultation and frequent assessment of patients will help clinicians manage these difficult cases correctly.
Emphysema involves a weakened distal airway. The normal elastic recoil is significantly impaired and the alveolar walls are thin and weakened. Be diligent in managing patients with alveolar recruitment while avoiding pressure-induced lung trauma. As with the asthmatic, expiratory times must be monitored closely to ensure complete expiration and to avoid air trapping.
Congestive heart failure is becoming a common problem. Unfortunately, stress on an already weakened heart can lead to infarction, dysrhythmias, and reduced flow to multiple organ systems. Caring for the decompensated patient in acute pulmonary edema involves managing multiple problems at once. The first step is an appropriately set ventilator. Mechanical ventilation can reduce the stress on the heart by reducing preload and afterload. You can maximize oxygenation while avoiding absorption atelectasis. While the task of stabilizing a truly ill CHF patient can be daunting, recognizing the utility of the ventilator is your first step to providing the appropriate care.
1. "My patient's peak inspiratory pressure alarm kept beeping, so I decided to decrease the tidal volume."
Did you check the plateau pressure first? Plateau pressure represents the force required to overcome the lung's elastic recoil. It will increase with decreased compliance, overdistention of the lungs, or hyperinflation. It is important to note the difference between overdistention (too much tidal volume and subsequent alveolar stretching) and hyperinflation (trapped air in the lungs). If the plateau pressure did not change then the lung's recoil force did not change, and the ventilator alarmed for another reason. Peak inspiratory pressure (PIP) also reflects the total force required to flow air through the lungs. Increases in PIP without change in plateau pressure may be due to an obstruction to airflow or an increase in airway resistance. In this case, higher PIP levels would not indicate overdistention. Consider increased secretions (suction the airway), bronchospasm (give bronchodilators), and obstruction (check ETT placement).
2. "My patient's peak inspiratory alarm kept beeping, and I couldn't find the silent button."
What if the increase in PIP was the result of decreased compliance? If the plateau pressure increased to the same extent that peak pressure increased without a difference between the two (no change in resistance: R = PIP - Pplat/Flow) then decreased compliance is to blame. Factors that decrease compliance include alveolar collapse/atelectasis (measure auto-PEEP and apply PEEP), lung collapse (decompress the pneumothorax), overdistention of the lung (decrease tidal volume), air trapping (reduce I-time or rate), and right mainstem intubation (confirm with CXR).
Please compare pitfall 1 and 2. Note that the PIP alarm in pitfall 1 resulted from increased airway resistance whereas it represents an increase in plateau pressure due to decreased compliance in this example. While these events may appear similar and the alarms sound the same, understand that their etiologies and management are quite different.
3. "Since my asthmatic had extremely high inspiratory resistance, I chose to use a lower inspiratory flow rate."
While a high inspiratory flow may exaggerate the high resistance in asthma and result in increased peak inspiratory pressures, a short inspiratory time is necessary to allow for a prolonged expiratory time. The expiration phase of breathing must be extended so air trapping is avoided. A careful balance must be obtained between adequate inspiratory flow, PIP, and sufficient expiratory times. Longer E-times are necessary to minimize air trapping and hyperinflation. Setting the ventilator to deliver a high inspiratory flow rate shortens I-time (thus, longer exhalation time). This will increase inspiratory flow and increase PIP. The severe airway resistance seen in asthmatics actually prevents the complete transference of this pressure to the alveoli (Poiseuille's law) thus averting alveoli from experiencing the full effect of the PIP. Recall the difference between PIP and plateau pressures. If you raise the PIP, there may not be a corresponding change in the plateau pressure after you shorten the I-time and lengthen the expiration because you've reduced the auto-PEEP somewhat. As a result, the plateau pressure will tend to decrease. A high peak airway pressure is not necessarily dangerous unless it corresponds to a dangerously high transalveolar pressure (Pplat).
4. "After applying PEEP of 8 cm H2O to overcome auto-PEEP in the pressure control mode, my COPD patient became hypotensive. I simply turned off the PEEP since I figured it was the problem."
Understanding a ventilator patient interface often involves thinking through what is happening between the person and the machine.
1) In pressure control, tidal volumes vary in relation to compliance.
2) PEEP that corrects auto-PEEP will result in an increased functional residual capacity and compliance.
3) If pressure control remains the same, the tidal volume will increase along with this increase in compliance. (C = Δ Vol/Δ Press)
4) If the pressure control (PC) dial was originally set to achieve a target tidal volume of 500 mL, your patient may now get a higher VT of 800 mL as a result of improved compliance.
5) This increase in VT may cause overdistention and hyperinflation. This can result in decreased right ventricular output, cardiac output, and subsequently reduced blood pressure.
Therefore, before taking away the PEEP that was effective in recruiting collapsed alveoli, consider decreasing the PC setting to achieve the original desired target VT of 500 mL and assuring that Pplat remains < 30 cm H2O. The suspicion of dynamic hyperinflation should also prompt you to listen to the lungs and assess for auto-PEEP (air trapping).
The effects of PEEP on decreasing right ventricular output, cardiac output, and blood pressure directly relate to how PEEP changes total lung volume and intra-alveolar pressures (Pplat).
The question to keep in mind is to what extent did the PEEP change your patient's lung volumes and plateau pressure and to not simply assume the effect was a direct result of PEEP.
5. "The plateau pressure was < 30 cm H2O, so I was able to increase my patient's tidal volume to 15 mL/kg."
Normal physiologic tidal volume is 6-8 mL/kg PBW. So why push a VT between 12-15 mL/kg when a patient is sick? Clearly, if a healthy person does not require such a large tidal volume then it would be wise to avoid such a stress on our critically ill patients.
Always take care to treat your patient and not a number. In a severely compromised patient with low compliance lungs, plateau pressures of 40 cm H2O may only establish a VT of 5 mL/kg PBW and induce lung injury because of high alveolar pressures. The rule of thumb is to adjust VT up to 10 mL/kg PBW as long as plateau pressure is < 30 cm H2O and adjust respiratory rate to achieve desired minute ventilation.
6. "Even though my emphysema patient was bucking the vent, I was relieved to see the improvement in his mental status."
Your patient is more awake because he is miserable! While your goal of intubation may have been to protect your patient, the lack of sedation and agitation is harming him.
After intubation, it is often necessary to use smaller tidal volumes as a protective lung strategy in patients at risk for VILI. Forcing a low VT of 5 mL/kg (when necessary) on a patient without sedation can result in marked cardiovascular instability as the patient will be fighting for larger tidal volumes. This increases the patient's discomfort, oxygen consumption, and carbon dioxide production. This and agitation will make the patient more active.
7. "I wanted to check for auto-PEEP on my patient receiving 10 cm H2O of PEEP. After performing the expiratory occlusion maneuver on the 10 cm H2O of PEEP, I discovered there was no auto-PEEP present so I decreased it to 0 cm H2O."
The 10 cm H2O of PEEP was actually counter-balancing the auto-PEEP. If you fail to decrease the PEEP to zero before performing the expiratory hold maneuver, you would have measured 10 cm H2O of auto-PEEP instead. If the set PEEP overcomes auto-PEEP, the true amount of auto-PEEP will be falsely low. As a minimum, physiologic PEEP is considered to be between 5 cm H2O and 8 cm H2O.
8. "The peak pressure alarm still continued to beep despite all my efforts."
The mnemonic DOPE (tube Displacement, tube Obstruction, Pneumothorax, and Equipment failure) covers 4 of the most common causes of post-intubation hypoxia or other deterioration. Displacement: Check for endotracheal tube placement by auscultating the chest and watching for equal bilateral chest expansion. If the ETT has been pushed into the right mainstem, slowly withdraw it until equal chest expansion and breath sounds are present. If still in doubt, use a laryngoscope blade and visualize directly. Obstruction: Does the patient need suctioning? Secretions in the airway can plug the ETT. Another cause could be the ventilator tubing. Check the ventilator to ensure no kinking in the circuit. Pneumothorax: Absent breath sounds may indicate a pneumothorax. Treatment requires decompression.
Equipment: While unusual, this complication can occur. Decipher where the problem has occurred by systematically approaching the ventilator from the patient back to the machine. Under any suspicion of mechanical failure, the patient should be removed from the ventilator and manually bagged on 100% O2.
9. "My asthmatic developed hypotension on pressure control. He was only on 5 cm H2O of physiologic PEEP."
At the initiation of mechanical ventilation, asthmatics may require higher pressures to overcome increased resistance caused by bronchospasm, persistent airway secretions, plugging, and inflammation. However, as bronchodilators and steroid therapy lead to clinical improvement and bronchospasm and inflammation clears, your patients will require lower set airway pressures to ventilate the lungs appropriately.
If this improvement is not recognized and initial pressure settings are not decreased, these high intrathoracic pressures are transmitted to the mediastinum. The resulting compression of superior and inferior venae cavae leads to decreased cardiac output and hypotension. Since the increased tidal volume delivered led to the hypotension, the best management would be to reduce the tidal volume; in this case, do so by lowering the pressure control setting. Checking the plateau pressure to assure it is < 30 cm H2O should be done with any change in a patient's target tidal volume.
10. "My CHF patient remained hypoxic on 100% FIO2."
In acute pulmonary edema, a high FIO2 alone is ineffective in improving PaO2 in the presence of a large intrapulmonary shunt. The reason for the persistent hypoxemia is that there is no transmission of oxygen to the red blood cells perfusing collapsed alveoli. This condition is known as refractory hypoxemia. In this case, PEEP is needed to splint open the airways so more alveoli can participate in oxygenation.
Case #1: You intubate the young asthmatic and start her on pressure control ventilation. She is adequately sedated and paralyzed as you obtain your initial settings. Because of severe obstruction and prolonged expiratory phase, you have the following settings: FIO2 100% and PCAC to achieve target tidal volume of 400 mL. The inspiratory occlusion maneuver reveals an acceptable plateau pressure of 27 cm H2O. After initial PEEP of 5, the P-flex suggests you set the PEEP at 8 cm H2O. The patient is adequately sedated with ketamine and morphine and therefore tolerates a respiratory rate of 6 breaths per minute and a short inspiratory time with a prolonged expiratory time. With these settings, you get an ABG of pH 7.28, PaO2 85, and PaCO2 of 110. The nurses are uncomfortable and ask to increase the respiratory rate and oxygen. Instead, you recognize the role of permissive hypercapnia and lung protective strategies. The whistling of the continuous nebs comforts you as you arrange an ICU admission.
Case #2: Your frail COPD patient deteriorated soon after you stabilized the asthmatic. You wondered if this man would ever come off the vent as you deftly slid the 8.0 ETT through the cords. After airway stabilization, you set your ventilator as follows: PCAC, FIO2 100%, PIP 22 (corresponds to volumes between 600 cc and 800 cc), PEEP 14 (based on P-flex), sedated with midazolam drips and morphine, short inspiratory time with a prolonged expiratory time, and a respiratory rate of 8. This gives you an ABG of pH 7.40, PaO2 170, and PaCO2 of 30. You ask the respiratory therapist to reduce the FIO2 based on the oxygenation pleth (there is a good wave) and to reduce the PIP. She remarks that the plateau pressures are already below 30, but you explain that the corresponding volumes are a bit too large for a 60 kg man.
Case #3: Stabilizing this patient was a chore that involved management of her myocardial infarction, flash pulmonary edema, and new onset seizure (either hypoxic or a run of ventricular tachycardia). Her short neck, large size, and frothy pink sputum made intubation difficult. She ended up with a 7-0 ETT tube, volume control ventilation, FIO2 100%, set tidal volumes of 400 (corresponding plateau pressure 35), initial PEEP of 5, sedated with morphine and propofol, and a respiratory rate of 14. A P-flex shows an optimal PEEP of 15. You set this and notice, with satisfaction, that her plateau pressures come down as you recruit more lung volume. You adjust her tidal volumes up to 500 as you maintain her plateau pressures < 30. You also reduce your FIO2 to 50% to avoid absorption atelectasis.
















%20Emergency%20MEdical%20Practice.jpg)
Evidence-based medicine requires a critical appraisal of the literature based upon study methodology and number of subjects. Not all references are equally robust. The findings of a large, prospective, randomized, and blinded trial should carry more weight than a case report.
To help the reader judge the strength of each reference, pertinent information about the study, such as the type of study and the number of patients in the study, will be included in bold type following the reference, where available. In addition, the most informative references cited in this paper, as determined by the authors, will be noted by an asterisk (*) next to the number of the reference.
Andrea DeGiorgi; Michael White
August 1, 2008