Table of Contents
About This Issue
Skin and soft-tissue infections (SSTIs) are common in children. SSTIs range from minor self-limiting illnesses to severe life-threatening diseases, and emergency clinicians must be able to rapidly diagnosis and promptly manage these conditions to improve outcomes. An understanding of the features that can help distinguish SSTIs from other conditions is crucial to making the correct diagnosis. This issue reviews various etiologies and patient presentations of common SSTIs, discusses findings on the history and physical examination that can differentiate SSTIs from mimics, and provides evidence-based recommendations for management of SSTIs in the emergency department. In this issue, you will learn:
The most common bacterial pathogens that cause SSTIs
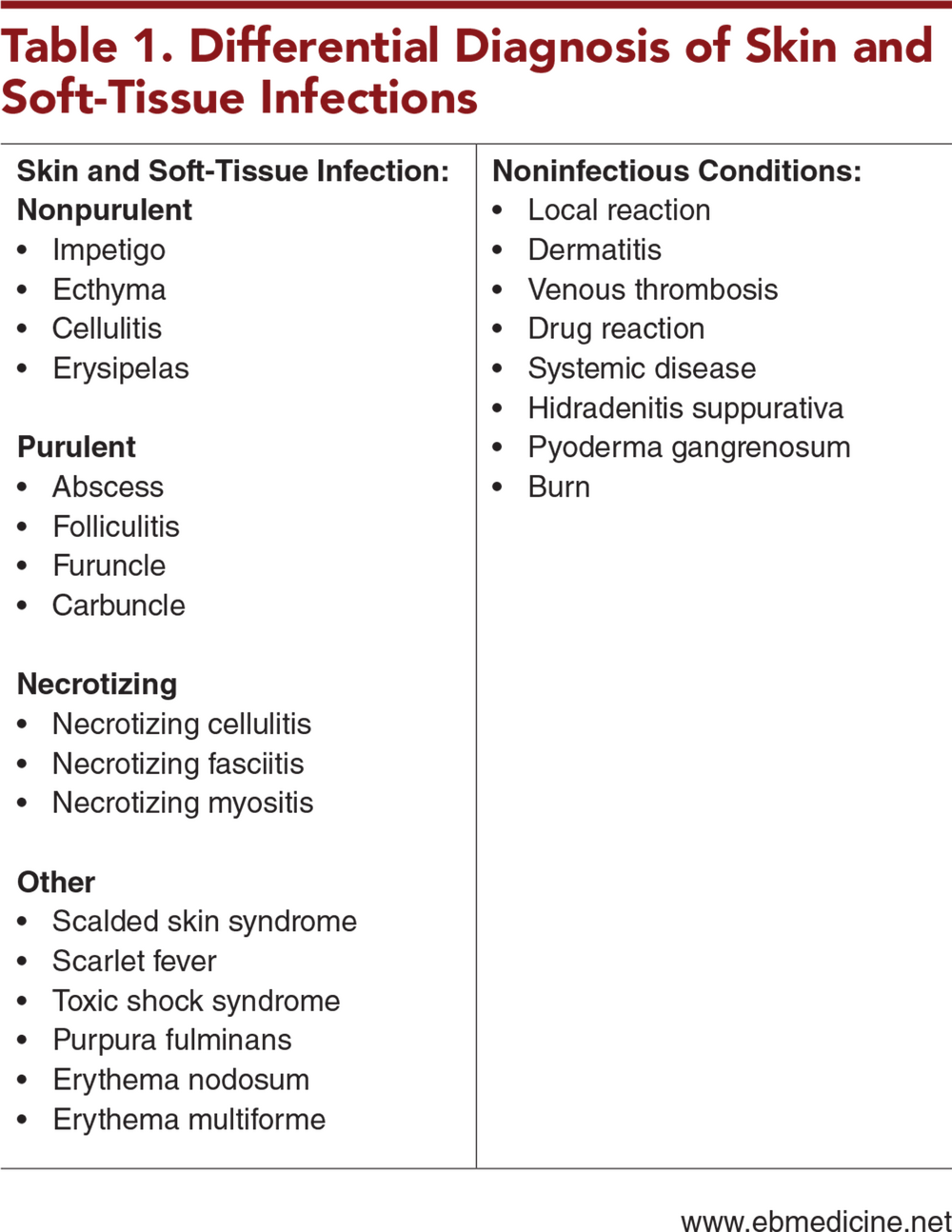
Conditions in the differential diagnosis of SSTIs
Key historical and physical examination findings that can narrow down the differential and help make the diagnosis
When wound or blood cultures are indicated
Which imaging studies can help identify cellulitis, abscess, and necrotizing fasciitis
Treatment recommendations for impetigo, erysipelas, nonpurulent cellulitis, abscess, bacterial folliculitis, furuncles and carbuncles, and necrotizing fasciitis
Recommendations for appropriate use of antibiotics to improve safety and reduce the cost of treating infections
Special considerations for treatment of bite wounds and exposures to specific organisms
Recommendations for decolonization for patients experiencing recurrent SSTIs
Which patients with SSTIs can be discharged safely with topical or oral antibiotics and close outpatient follow-up, and which patients should be admitted
- About This Issue
- Abstract
- Case Presentations
- Introduction
- Critical Appraisal of the Literature
- Etiology and Pathophysiology
- Anatomy
- Pathogenesis
- Differential Diagnosis
- Nonpurulent Infections
- Purulent Infections
- Necrotizing Infections
- Hidradenitis Suppurativa
- Prehospital Care
- Emergency Department Evaluation
- History
- Physical Examination
- Diagnostic Studies
- Laboratory Studies
- Imaging Studies
- Treatment
- Nonpurulent Infections
- Impetigo
- Erysipelas
- Nonpurulent Cellulitis
- Purulent Infections
- Abscess
- Incision and Drainage
- Antimicrobial Therapy
- Bacterial Folliculitis
- Furuncles and Carbuncles
- Necrotizing Infections
- Necrotizing Fasciitis
- Other Considerations
- Hidradenitis Suppurativa
- Bite Wounds
- Specific Exposures
- Special Populations
- Immunocompromised Patients
- Infants Aged <60 Days
- Controversies and Cutting Edge
- Decolonization
- Health Equity
- Disposition
- Summary
- Risk Management Pitfalls in Emergency Department Management of Skin and Soft-Tissue Infections
- 5 Things That Will Change Your Practice
- Time- and Cost-Effective Strategies
- Case Conclusions
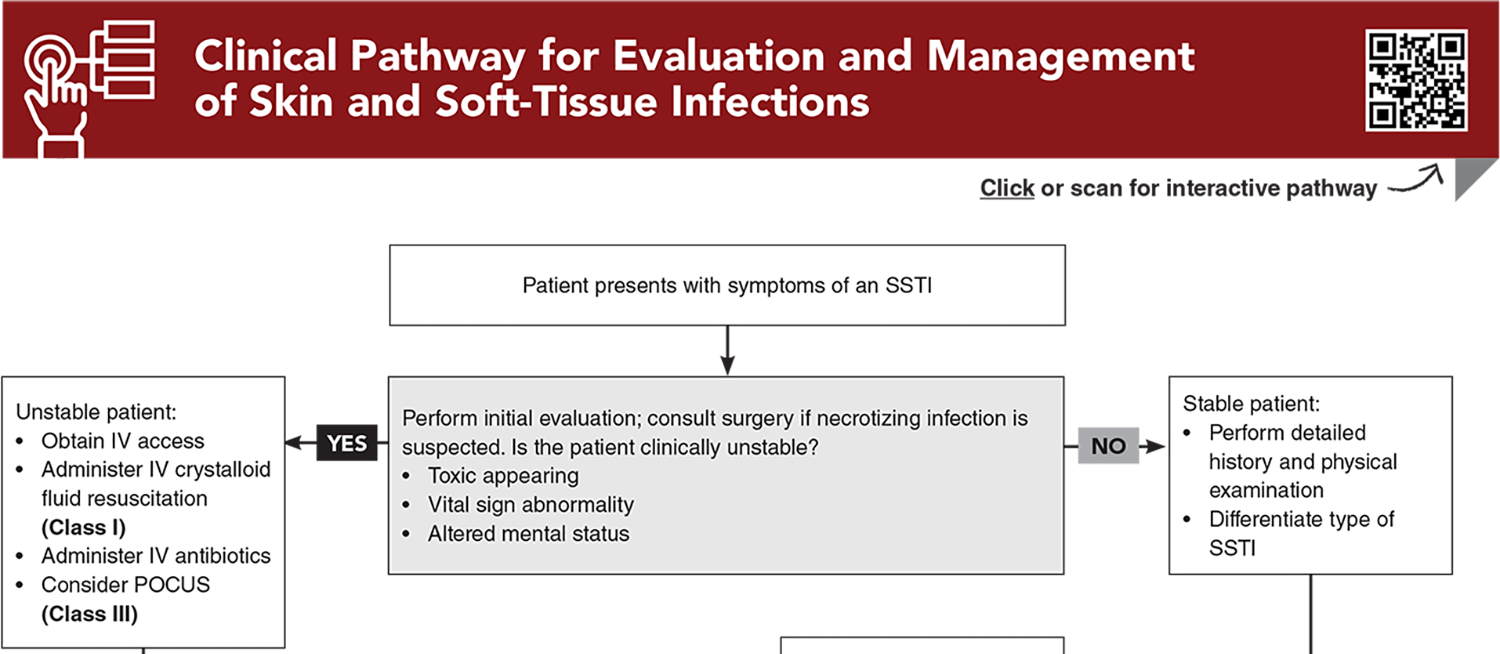
- Clinical Pathway for Evaluation and Management of Skin and Soft-Tissue Infections
- Tables and Figures
- References
Abstract
Children with skin and soft-tissue infections (SSTIs) commonly present to the emergency department, and while most of these infections are mild, some can be severe, with high morbidity and mortality. Emergency clinicians must be able to recognize frequently encountered SSTIs and be prepared to treat them appropriately. This issue reviews the various etiologies and patient presentations of common SSTIs, including purulent, nonpurulent, and necrotizing infections. Findings on the history and physical examination that can differentiate SSTIs from mimics are discussed, and evidence-based recommendations are provided for management.
Case Presentations
- He is accompanied by his mother, who recalls that he had a barely noticeable scratch on the arm after playing at the playground a few days ago.
- His mother says redness developed and extended gradually over the next 3 days.
- On examination, he has a 4-cm area of erythema that is mildly tender to palpation. His history and vital signs confirm no fever or other concerning systemic signs.
- Are laboratory studies and imaging indicated? Should you prescribe antibiotics?
- Her parents say this started as a smaller area of redness and swelling that has grown larger over the last 4 days. They have not seen any drainage in the area but have noticed it is rather painful. They tell you the girl has not had any fevers, and she is active but fussy.
- The girl’s vital signs are normal. On examination, you note a 7-cm area of erythema and induration, but it is unclear whether it is fluctuant. There is no obvious drainage or pustule. The girl cries during the examination, and it appears to be related to tenderness.
- How should you further evaluate this patient? What imaging should you consider?
- The boy’s mother tells you he fell from his bike 2 days ago and sustained an abrasion, but otherwise, he seemed fine at the time. The family tells you they were alarmed that within 24 hours after the fall, the boy developed significant redness, swelling, and pain. They planned to take him to their pediatrician but became worried when he did not appear well and became significantly less active, which prompted the call to EMS.
- The boy’s vital signs are: temperature, 39.0°C; heart rate, 140 beats/min; blood pressure, 88/40 mm Hg; respiratory rate, 18 breaths/min; and oxygen saturation, 98% on room air.
- What are the most important and immediate next steps? Who should you consider consulting?
How would you manage these patients? Subscribe for evidence-based best practices and to discover the outcomes.
Clinical Pathway for Managing Patients Presenting with Acute Diarrhea in Urgent Care
Subscribe to access the complete Clinical Pathway to guide your clinical decision making.
Tables and Figures
Subscribe for full access to all Tables and Figures.
Buy this issue and
CME test to get 4 CME credits.
Key References
Following are the most informative references cited in this paper, as determined by the authors.
9. * Sutter DE, Milburn E, Chukwuma U, et al. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics. 2016;137(4):e20153099. (Retrospective; 39,207 patients) DOI: 10.1542/peds.2015-3099
71. * Daum RS, Miller LG, Immergluck L, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. New Engl J Med. 2017;376(26):2545-2555. (Randomized controlled trial; 786 patients) DOI: 10.1056/NEJMoa1607033
92. * Glenn IC, Bruns NE, Craner D, et al. Same-day discharge after incision and drainage of soft-tissue abscess in diaper-age children is safe and effective. Pediatr Surg Int. 2017;33(5):601-604. (Retrospective; 408 patients) DOI: 10.1007/s00383-017-4070-y
99. * Gottlieb M, Avila J, Chottiner M, et al. Point-of-care ultrasonography for the diagnosis of skin and soft tissue abscesses: a systematic review and meta-analysis. Ann Emerg Med. 2020;76(1):67-77. (Systematic review, meta-analysis; 14 studies, 3592 patients) DOI:10.1016/j.annemergmed.2020.01.004
Subscribe to get the full list of 105 references and see how the authors distilled all of the evidence into a concise, clinically relevant, practical resource.
Keywords: skin infection, soft-tissue infection, skin and soft-tissue infection, SSTI, necrotizing infection, purulent infection, nonpurulent infection, cellulitis, impetigo, ecthyma, erysipelas, abscess, folliculitis, furuncle, carbuncle, necrotizing cellulitis, necrotizing fasciitis, necrotizing myositis, Staphylococcus aureus, MRSA, S aureus, Streptococcus pyogenes, S pyogenes, GAS, dermatitis, hidradenitis suppurative, incision and drainage, loop drainage, bite wounds, decolonization