
Patients who present with a supraventricular tachycardia (SVT) may have potentially life-threatening disease, and their outcome is often directly related to the care they receive in the emergency department (ED). In a matter of minutes, the emergency physician must quickly and confidently assess and support the “ABC’s” and determine the need for interventions. The search for an underlying condition must be initiated while immediately addressing the patient’s stability. Synchronized cardioversion is often indicated for unstable patients, while a more sophisticated approach is needed to decipher and manage the stable patient. Although SVTs are a frequent cause of ED1 and primary care office2 visits, they are infrequently the primary reason for hospital admission.1-3 This issue of Emergency Medicine Practice will provide a review of SVTs with a focus on the ECG analysis and the evidence behind the most recent ACLS guidelines.4 The topics of atrial flutter and atrial fibrillation will not be reviewed in detail in this issue as they were the focus of a previous Emergency Medicine Practice issue.5
A 35-year-old female suddenly develops palpitations and chest discomfort while white-water rafting. Paramedics arrive and record a HR of 190 bpm. She is immediately transported to the local emergency department where you are working. On exam she appears anxious and has a regular HR at a rate of 75 bpm. An ECG, basic labs, and a urine toxicologic screen are ordered. An hour later the toxicologic screen and basic labs come back negative. You are about to discharge her with a diagnosis of acute anxiety attack when you realize that you haven't seen her ECG yet...
Soon after, you see paramedics rolling a gurney with an older male into another room. They give you the quick report that the patient is having intermittent shortness of breath and chest discomfort. You look at the vital signs on the run sheet and are reassured to see that his blood pressure is 140/80 with an O2 sat of 98% on room air. You ask the nurse to place the patient on a monitor while you take care of a few other things. Minutes later, you are paged overhead to his room and the nurse tells you, "His heart rate is 180/min and he really doesn't look good." The patient is diaphoretic and his BP is now 100/50. You ask the patient how he is feeling and he mumbles a response that is barely omprehensible. The monitor shows a narrow- complex tachycardia. An ECG is obtained and no P waves are discernible. You're on the fence; can you convert this SVT with adenosine or should you cardiovert?
A literature search was conducted in the following databases: Ovid MEDLINE, EMBASE, the Cochrane Library (including the Cochrane Database of Systematic Reviews and the Cochrane Controlled Trials Registry), and Best Evidence. Searches were limited to English language sources and to human subjects.
Medication recommendations follow the format of American College of Cardiology/American Heart Association (ACC/AHA) guidelines for classifying indications, summarizing both the evidence and expert
opinion (Table 1).

The term "supraventricular tachycardia" is imprecise and commonly refers to a spectrum of paroxysmal tachydysrhythmias that require sinus nodal, atrial, AV (atrioventricular) nodal tissue, or a combination of these tissues for their commencement and continuation. For the purposes of this review, the term SVT refers to the following diagnoses: AV reentry tachycardia (AVRT), AV nodal reentry tachycardia (AVNRT), unifocal atrial tachycardia (UAT), multifocal atrial tachycardia (MAT), sinoatrial reentry tachycardia (SNRT), and junctional tachycardia (JT). In this review, atrial fibrillation and atrial flutter are not included in the calculation of statistics or discussion related to the general term SVT. Misdiagnosis and inconsistent classification prevent accuracy of the exact prevalence of SVT; however, it is estimated to affect about 225 individuals per 100,000 and has an estimated incidence of approximately 35 cases per 100,000 persons per year, with half of all new cases diagnosed in the ED.6 Nationally, SVT accounts for approximately 50,000 ED visits per year.1 The epidemiology of supraventricular rhythms varies with age, gender, and associated comorbidity. In a large cohort of patients in the Midwest United States, the average age at the time of SVT onset was 57 years (ranging from infancy to more than 90 years of age).6 Female patients in this database had a two-fold greater relative risk (RR 2.0, 95% confidence intervals 1.0-4.2) of developing SVT compared to men.6 Additionally, 86% of patients older than 50 years of age reported underlying cardiovascular disease. Compared to those with underlying cardiovascular disease, those without underlying disease (i.e., "lone" SVT) were younger (mean age 37 years vs. 69 years), had faster heart rates (186 bpm vs. 155 bpm), and were more likely to present first to an ED (69% vs. 30%).6 Whereas half of all females who present with SVT have lone SVT, only 10% of male patients are noted to have lone SVT.6
Supraventricular tachydysrhythmias are produced by disorders of impulse formation and/or disorders of impulse conduction. The former are referred to as automatic, the latter as reentrant. Cells with enhanced automaticity exhibit enhanced diastolic depolarization and, therefore, can usurp the sinus node to become the predominant pacemaker of the heart. The rapid firing rate can be classified as incessant (i.e., more than 50% of the day) or episodic. Cardiac tissue in the atria, AV node, common His bundle, or vascular tissue feeding the atria (such as the pulmonary veins or vena cava) may all exhibit enhanced automaticity.7-9 Examples of SVT due to an enhanced automaticity mechanism include certain types of unifocal atrial tachycardia and nonparoxysmal junctional tachycardia (NPJT).
Most types of supraventricular tachycardia have a reentry mechanism. In its simplest form, reentry occurs as repetitive excitation of a region of heart that begins as a premature discharge from an ectopic supraventricular focus and propagates through conduction around a functional or fixed obstacle in a defined circuit. These rhythms are classified according to the location of the reentry circuit. AVNRT and AVRT are two classic examples of reentry SVT. Approximately 60% of SVT cases are due to an AV nodal reentry circuit, and approximately 30% are due to an AV reentry circuit mediated by an accessory pathway. Sinus nodal reentry and intra-atrial tachycardias are also examples of supraventricular tachycardia with a reentry mechanism, but both are exceedinglyuncommon.
In patients that are electrophysiologically prone to AVNRT, the AV node is essentially divided into two pathways that form a reentry circuit and then share a final common pathway through the lower AV node and common His bundle (Figure 1). One of the pathways has a fast depolarization time but slow repolarization time (β pathway), whereas the other has a slower depolarization and a faster repolarization time (α-pathway). Normally, antegrade conduction occurs through the fast pathway and the second pathway is not invoked. When a premature atrial depolarization occurs, it finds the fast pathway inaccessible due to its slower repolarization time. The impulse is then carried antegrade through the slower pathway. After travel through this pathway, a portion of the impulse conducts through to the ventricles but also conducts retrograde through the fast pathway (i.e., with slow-fast or typical AVNRT, conduction occurs antegrade through the slow limb and retrograde through the fast limb). A loop is created when the retrograde portion of the impulse finds the slower pathway fully repolarized and ready to accept another depolarization. Atypical AVNRT occurs less than 10% of the time and is seen when either a slow-slow pathway or a fast-slow pathway occurs. The fast-slow pathway is thought to be anatomically the same as the slow-fast pathway, but in reverse.10 In cases of both typical and atypical AVNRT, the QRS duration is < 120 msec in the absence of aberrant conduction or bundle branch block (BBB).

Unlike AVNRT, patients with AVRT have a normal AV node with a single pathway for conduction but have an accessory pathway capable of impulse propagation (Figure 2). The archetypal example of this occurs in patients with the Wolff-Parkinson-White (WPW) syndrome who have an accessory pathway termed the bundle of Kent. Normally, antegrade conduction occurs through both the bundle of Kent and AV node nearly simultaneously. The characteristic findings of WPW syndrome during sinus rhythm include a short PR interval (due to rapid conduction through the Kent bundle) and a δ wave and widened QRS (due to depolarization of ventricular myocardium adjacent to the terminal ventricular aspect of the Kent bundle). As soon as conduction through the AV node occurs, rapid depolarization of the bulk of the ventricular myocardium occurs.
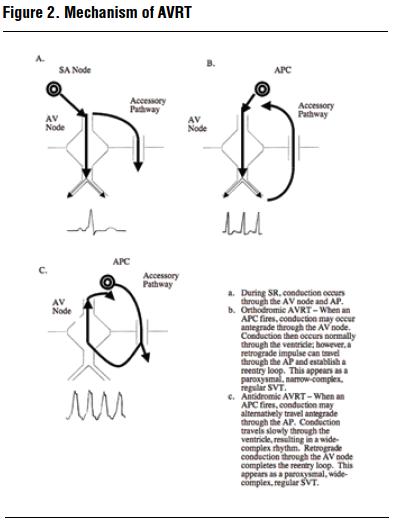
When a premature atrial depolarization occurs, it typically finds the bundle of Kent in a refractory state and travels antegrade through the AV node. After travel through this pathway, a portion of the impulse conducts through the ventricles but can also conduct retrograde through the bundle of Kent. A loop is created when the retrograde portion of the impulse finds the AV node fully repolarized and ready to accept another impulse. Conduction that follows the above course is termed orthodromic (Greek: ortho - straight; dromic - course) and appears as a narrow-complex regular tachycardia in the absence of BBB.
In antidromic (Greek: anti - against; dromic - course) AVRT conduction, the reentry circuit travels antegrade through the accessory pathway (i.e., against the normal course to the ventricles). Depolarization of the heart occurs slowly, since the bundles and purkinje fibers are not involved in depolarization of the ventricular myocardium. This results in a widecomplex regular tachycardia that is typically indistinguishable from ventricular tachycardia. Retrograde conduction through the AV node completes the reentry circuit. Antidromic AVRT is rare, occurring in only 5-10% of people with WPW syndrome.11,12
The general approach to differentiate SVTs is based on ECG findings. In essence, dysrhythmias should initially be divided based on QRS width (i.e., < 120 msec vs. ≥ 120 msec) and regularity of the underlying rhythm (i.e., regular RR intervals vs. irregular RR intervals). Characterization of the P wave should then allow for accurate identification of the underlying dysrhythmia (Table 2).

In the absence of aberrant conduction or BBB, the vast majority of SVTs present with ECG evidence of a narrow-QRS complex (i.e., < 120 msec duration). Examples of narrow-complex regular tachydysrhythmias include sinus tachycardia (ST), AVNRT, orthodromic AVRT, unifocal atrial tachycardia, junctional tachycardia, and atrial flutter with fixed rapid conduction. Narrow-complex irregular tachydysrhythmias include atrial fibrillation with rapid ventricular response, atrial flutter with rapid and variable conduction, multifocal atrial tachycardia, and sinus tachycardia with multiple premature atrial contractions (PACs).
A SVT in the presence of a BBB or with antegradeconduction through an accessory pathway may exhibit a wide QRS complex (> 120 msec duration). Examples of wide-complex SVTs include antidromic AVRT and any narrow-complex rhythm with a preexisting or rate-related BBB (e.g., SVT with right BBB [RBBB]). Additionally, the differential diagnosis for wide-complex tachydysrhythmias includes rhythms that originate from below the bifurcation of the common His bundle (e.g., ventricular tachycardia, ventricular fibrillation, fascicular tachycardia).
Appropriate (physiologic) sinus tachycardia is defined as a heart rate of greater than 100 bpm (typically 100-160 bpm, but may be as high as 220 bpm)13 in response to physical, emotional, pathologic, or pharmacologic stimulus.
In the ED, sinus tachycardia should be regarded as a marker of an underlying condition (Table 3). The mechanism responsible for sinus tachycardia is thought to result from physiologic influences on pacemaker cells and from an anatomic shift of the site of depolarization within the sinoatrial node.14 Inappropriate sinus tachycardia (IST) is a persistently elevated resting heart rate (≥ 100 bpm) or sinus rate that is excessive compared to the physical, emotional, pathological, or pharmacological stress. The diagnosis of IST should only be made in consultation with a cardiologist and after performing a comprehensive evaluation to exclude typical causes of sinus tachycardia. Postural orthostatic tachycardia syndrome (POTS) is seen in patients without autonomic neuropathy and occurs when an exaggerated persistent postural sinus tachycardia (greater than 30 bpm from baseline or greater than 120 bpm) occurs when the patient moves from the supine to the upright position in the absence of postural hypotension.

Sinus tachycardia (Figure 3) is characterized by a non-paroxysmal narrow-complex regular tachycardia with P waves of normal contour and axis preceding QRS complexes at a fixed PR interval. On an ECG, the P wave should be negative in AVR and positive in leads I, II, III, and AVF. It can be negative in leads V1 and V2 but positive in leads V3 to V6 because, in the horizontalplane, it is conducted anteriorly and slightly leftward.

SNRT is a rare tachycardia (less than 5% of SVT) that is difficult to diagnose with 12-lead ECG.15 In SNRT, a premature supraventricular beat triggers the abrupt onset of circus movement within a reentry loop of conduction tissue within the sinus node itself. A patient with SNRT will appear to display sinus tachycardia on their ECG; however, 1) there is an abrupt onset to this rhythm, 2) there is often no apparent underlying etiology to the tachycardia, and 3) there is abrupt termination of this rhythm with vagal maneuvers or adenosine.
During SNRT, the P waves may be identical to those in sinus rhythm but are more likely to exhibit subtle differences that may be difficult or impossible to discern on surface ECG. The RP interval is usually longer than the PR interval.16 Paroxysms of SNRT are frequently triggered and terminated by a premature atrial stimulus.
AVNRT is the most common form of SVT, accounting for 60% of all cases of SVT.17 It is more prevalent in females and has an average age of tachycardia onset of 32 years +/- 18 years.18,19 AVNRT is typically not associated ith underlying cardiovascular disease. The characteristic heart rate is between 118 and 264 bpm (mean 181 +/- 35 bpm).20
AVNRT is a paroxysmal narrow-complex regular tachycardia that generally does not have a clearly identifiable P wave that precedes the QRS complex (Figure 4). In typical AVNRT (90% of AVNRT), retrograde atrial activation can occur simultaneously with ventricular activation, resulting in a retrograde P wave that is superimposed on or immediately follows the QRS complex; this results in a short RP interval. If the P wave follows the QRS complex, this appears as a pseudo S wave (present during AVNRT but not during sinus rhythm) in leads II, III, and aVF and as a pseudo R wave in lead V1. Occasionally, the P wave may be completely buried within the QRS complex and not visible on the 12-lead ECG.21 The presence of a pseudo S, a pseudo R, or both is 90-100% specific and has an 81% positive predictive value for typical AVNRT.22,23

However, these criteria are only 42% sensitive for this diagnosis.22 ST segment depression (≥ 2 mm) is not uncommon and may result from changes in ventricular repolarization due to abrupt cycle length shortening and/or retrograde atrial activation. The presence of ST segment depression in this setting is not indicative of myocardial ischemia.24,25
In atypical AVNRT (10% of AVNRT), retrograde atrial activation may occur long after ventricular activation, producing a long RP interval. A P wave that precedes the QRS can be seen with atypical AVNRT and is usually negative in leads II, III, and aVF and positive in leads V1, V2, I, aVR, and aVL.20,26 Differentiating between typical and atypical AVNRT on the 12-lead ECG is not necessary for successful treatment in the emergency department.
AVRT is the second most common form of SVT, occurring in 30% of all cases of SVT.17 The average age of symptom onset is 23 years +/- 14 years.18,19 The typical heart rate is between 124 and 256 bpm (mean 183+/- 32 bpm).20 Patients with AVRT have an anatomic accessory pathway that electrically connects the atria directly with the ventricle, bypassing the AV node. ECG evidence of an accessory pathway (a delta wave) is reported to be present in 0.15-0.25% of the general population.27 However, the prevalence more than triples in first degree relatives of patients with an accessory pathway and evidence of preexcitation.28 AVRT is the most common arrhythmia that occurs in patients with an accessory pathway, accounting for 95% of SVTs in this group.
Nonsustained UAT is frequently seen on cardiac monitoring but is rarely associated with symptoms. Sustained UAT is seen in 10-15% of patients referred for catheter ablation of SVT.34 UAT accounts for 10-23% of SVT in children with normal hearts and a much higher percentage in those with congenital heart disease (both repaired and unrepaired).35-38 Onset of symptoms may occur at any age, but the majority of patients experience their first episode before age 40.19 In adults, underlying cardiovascular disease is often present.11
UAT is a paroxysmal narrow-complex regular tachycardia that is characterized by a non-sinus appearing single morphology P wave that fires at a rate between 100 and 250 bpm. Frequently, the P wave is obscured by the T wave of the preceding QRS complex and one is able to cherchez le P on let T (French - "search for P wave on the T wave").39 The presence of an isoelectric baseline and atrial rate < 250 bpm assists in differentiating UAT from atrial flutter with rapid ventricular response, and the presence of AV block during tachycardia excludes AVRT and makes AVNRT very unlikely.11 Whereas UAT caused by abnormal automaticity often exhibits a "warm-up" phenomenon in which the heart rate gradually increases over 3-4 beats before reaching a fixed peak, UAT caused by microreentry is paroxysmal and initiated by a premature atrial beat. UAT with AV nodal block can be seen with digoxin poisoning or with organic heart disease.40
JT arises from the AV node or common His bundle and can be paroxysmal or nonparoxysmal. Paroxysmal junctional tachycardia (PJT), also known as focal, automatic, or ectopic junctional tachycardia, is seen in two distinct populations: 1) infants and children with congenital heart abnormalities, and 2) young adults often related to stress or exercise.41 Nonparoxysmal junctional tachycardia (NPJT) results from enhanced automaticity arising from a high junctional focus 42 or in response to a triggered mechanism.43 It can be seen with acute myocardial infarction (usually inferior myocardial infarction), hypokalemia, chronic obstructive lung disease with hypoxia, post-valvular cardiac surgery or coronary artery bypass graft, myocarditis, and digitalis toxicity.11 In the immediate cardiac surgery post-operative period, NPJT may develop from AV nodal inflammation or trauma.44
JT is a narrow-complex regular tachycardia. Whereas PJT exhibits a ventricular rate in the 110-250 bpm range, NPJT rarely exceeds a ventricular rate of 120 bpm. ECG manifestations can be variable depending on the location of the junctional focus. The P wave may precede, be buried in, or follow the QRS complex. The QRS complex is usually narrow, but can be wide if BBB or aberrancy is present. In PJT, the relationship between the atrial and ventricular rates may vary. If retrograde (ventricular-atrial) block is present, the atria remain in sinus rhythm, and AV dissociation will be present. If retrograde atrial activation (inverted P waves in II, III, and aVF) occurs, a constant QRS-P interval is usually present. In NPJT, there is commonly one-to-one AV association. Digitalis toxicity should be considered if atrial fibrillation is present, but the ventricular response is narrow-complex and regular (i.e., atrial rhythm: atrial fibrillation; AV node: complete heart block; ventricular rhythm: junctional tachycardia). Anterograde AV nodal Wenckebach conduction block may also be seen with digoxin toxicity.45
Atrial flutter is caused by a rapid macroreentrant circuit occurring in the atria and is characterized by a regular atrial rate of 250-350 bpm. Ventricular conduction can either be fixed (e.g., 2:1 atrial:ventricular activations) or variable. Atrial flutter with fixed rapid conduction is a narrow-complex regular tachycardia (Figure 9).

Commonly there is 2:1 fixed AV block and the ventricular rate is approximately 150 bpm. Indeed, patients that present with a narrow-complex regular tachycardia with a rate of approximately 150 bpm should be initially presumed to be in atrial flutter with 2:1 block, until this diagnosis is excluded. Unlike atrial tachycardia, in atrial flutter, 1) the atrial rate is approximately 300 bpm, and 2) rapid regular atrial undulations (flutter or "F" waves) prevent identification of an isoelectric baseline. Inverted flutter waves are best seen in leads II, III, and aVF which give rise to the "picket-fence" or "sawtooth" appearance of this atrial dysrhythmia. Atrial flutter with 1:1 conduction generally propagates aberrantly due to the rapid rate and appears as a wide-complex rhythm. Although rare, when present, 1:1 conduction may result in significant hemodynamic compromise.
Atrial flutter with rapid but variable ventricular conduction can appear as an irregular tachycardia on ECG (Figure 10). It can be distinguished from atrial fibrillation and MAT by the presence of flutter waves of uniform consistency in leads II, III, aVF, or V1.

The atrial activity in atrial fibrillation is totally irregular and represented by fibrillatory (f) waves of varying amplitude, duration, and morphology causing random oscillation of the baseline. P waves are absent (Figure 11). The ventricular rhythm is typically irregularly irregular; however, it can be regular if third-degree AV block is present. The atrial rate is between 400 and 600 bpm and the ventricular rate is usually 100-180 bpm. If the ventricular rate without AV blocking drugs is less than 100 bpm, AV conduction disease is likely to be present. The WPW syndrome should be considered if the ventricular rate is > 250 bpm and the QRS is > 120 msec.

Premature complexes can introduce irregularity into an otherwise regular rhythm. The result of the insertion of PACs into a sinus tachycardia is a narrow-complex tachycardia that appears irregular. However, scrutiny of the ECG will reveal one dominant pacemaker (i.e., the sinus node), which helps distinguish this rhythm from MAT. Each premature atrial impulse has one of three fates. The P wave may be normally conducted, aberrantly conducted, or not conducted at all.
Prehospital providers who encounter a tachydysrhythmia should pay particular attention to support of the ABC's and should transmit a 12-lead ECG, if possible, to the receiving hospital. A targeted history and physical examination (including a complete medication list and social history with inquiry regarding recreational drug use) should be obtained.
There is limited data supporting the out-of-hospital use of AV nodal blocking agents for the treatment of
SVT. Several small prehospital SVT studies completed in the 1990's reported sinus conversion rates between 65% and 90% using either adenosine or verapamil.47-54 In general, these reports confirm that these agents are well tolerated and are relatively benign even when given inappropriately. There are no primary reports describing the use of beta-blockers or cardioversion inthe prehospital setting for SVT.
A substantial issue in the emergency medicine literature is the misidentification of supraventricular rhythms and the inappropriate administration of AV nodal blocking agents.47-49,51-53,55 In general, both adenosine and verapamil are well tolerated; however, rare cases of cardiovascular collapse have been described when AV nodal blocking agents have been given to critically ill patients with atrial fibrillation 56 or to patients with wide-complex tachydysrhythmias.51 A 10-year review of the accuracy of paramedic recognition and treatment of SVT found that inappropriate use of adenosine occurred in 20% of cases.55 An initial heart rate < 160 bpm and the absence of a medical history of either fast heart rate or palpitations were associated with inappropriate use of adenosine. Misidentification
rates were reduced to less than 10% following a targeted education program on tachydysrhythmias.
Arrhythmia-related symptoms can include a sense of chest fluttering or palpitations, chest discomfort, dyspnea, tiredness, fatigue, lightheadedness, presyncope, or rarely syncope. However, patients
with paroxysmal symptoms often are asymptomatic between episodes. Of equal importance in medical
decision making is a clinical history describing the pattern of symptoms in terms of the number of episodes, duration of individual episodes, frequency of recurrence, and medication, food-related, or physiologic triggers. Clinicians should ask about possible triggers, including caffeine and alcohol intake, drug use, and medication changes. SVTs usually do not have identifiable physical triggers. However, in a small number of people, maneuvers that alter autonomic tone, such as bending over or neck pressure, may precipitate the SVT.
If palpitations are part of the symptomatology, it is helpful if the patient is able to categorize them as regular or irregular. Palpitations are often due to premature ventricular contractions and register as irregular beats in an otherwise normal pulse. Sinus tachycardia may present as regular palpitations with a gradual onset and termination. If sinus tachycardia is suspected, additional history inquiring about possible stressors (e.g., fever, anxiety, medications) or volume loss should be elicited. Regular palpitations with an abrupt onset and termination are consistent with SVT. The diagnosis of SVT is further suggested by termination of the palpitations by vagal maneuvers such as coughing orbowel movements. Perceived irregular palpitations may be due to premature or escape beats, atrial fibrillation, or MAT.
Syncope is an uncommon presenting symptom of SVT. When present, it is often precipitated by either
initiation of a rapid SVT or a prolonged pause after termination of the SVT. If there is a history of syncope, it is important to consider atrial fibrillation with rapid conduction over an accessory AV pathway or structural heart disease as diagnostic possibilities. More often, syncope is due to acute vasodilation and/or rapid heart rate with low cardiac output.57
History taking should explore the possibility of hyperthyroidism, specifically asking about increased
bowel movements, trembling hands, weight loss, insomnia, and increased nervousness/anxiety. Additional history should focus on anxiety symptoms, as patients who present with palpitations may have been previously misdiagnosed with panic disorder without appropriate evaluation for SVT.
Most often, the physical examination is unremarkable and does not contribute significantly to the diagnosis of an SVT. Nonetheless, the physical examination may provide clues as to the diagnosis, cause, or predisposing condition associated with the SVT. The initial priority in assessing the patient should be evaluation of the vital signs and ensuring that the patient is hemodynamically stable. Subsequently, signs of volume status should be noted, including skin turgor and appearance of mucus membranes. This data can be helpful in identifying hypovolemia as a cause of sinus tachycardia. Additionally, temperature should be noted as fever may contribute to sinus tachycardia.
If the physical examination is performed during an episode of SVT, prominent jugular venous A
waves may be seen due to atrial contraction against a closed tricuspid valve; this finding is referred to as the "frog sign."17,58 Examination of the thyroid for bruits, enlargement, and nodules is part of a thorough examination. The finding of pale conjunctiva may be indicative of anemia. Physical examination may also identify signs of underlying cardiovascular disease that would predispose the patient to SVT or other arrhythmias. Mitral valve prolapse is often associated with supraventricular arrhythmias and palpitations; it is characterized by a midsystolic click auscultated during physical examination. Lower extremity edema, jugular venous distension, an S3, and rales may be present in congestive heart failure. A laterally displaced PMI (point of maximal impulse) may be indicative of left ventricular hypertrophy and hypertension. Evidence of chronic lung disease should also be documented on physical examination.
The diagnostic yield of routine laboratory tests (e.g., complete blood count, serum electrolytes) for patients with supraventricular tachydysrhythmias is low.17
A urine toxicologic screen can be useful to diagnose patients with persistent, unexplained tachycardia. Sympathomimetics, such as cocaine or amphetamines, and anticholinergic agents are common causes of tachycardia.59,60
Hyperthyroidism has a well-defined link to sinus tachycardia and atrial fibrillation with rapid ventricular response, but it can also present with SVT, MAT, or atrial flutter.61
Common insight may theorize that tachycardic rhythms lead to demand myocardial ischemia, requiring the ordering of cardiac troponins for evaluation of myocardial damage. However, SVT can lead to false positive troponin results62,63and may lead to inappropriate anti-anginal therapies or coronary angiography. For this reason, cardiac enzyme testing should not be performed based on the presence of rapid heart rate alone; instead, troponin testing should be sensibly ordered when a patient's symptoms or presentation are consistent with ischemic heart disease. Clinicians should consider the possibility of myocardial ischemia as the precipitating factor in arrhythmias such as NPJT and MAT, but further history taking and review of the ECG should guide the ordering of cardiac enzymes. Interpretation of a marginally elevated troponin is best done in consultation with a cardiologist.
There is an association between cardiomegaly and supraventricular arrhythmias; however, the results of chest radiography do not usually assist with either the diagnosis or treatment of SVT. Exceptions exist when the CXR identifies underlying disease precipitating SVT (e.g., pneumonia, pulmonary edema/congestive heart failure, pneumothorax). As such, a CXR may be reasonable to obtain in the patient with their first episode of SVT, but it is not required in patients with recurrent, isolated episodes of SVT in the absence of new or worsening pulmonary symptoms.
A resting 12-lead ECG should be obtained following initial stabilization of the patient with SVT. The presence of an abnormal rhythm, preexcitation, prolonged QT interval, sinus tachycardia, ST segment abnormalities, or evidence of underlying heart disease should be noted. Review of the ECG should not be limited to SVT-related findings but should also include specific evaluation for life threatening conditions such as evidence of Brugada syndrome64 or Wellens syndrome.65Increasing the ECG paper speed to 50 mm/sec from 25 mm/sec improves its diagnostic accuracy for SVT. In a review of the diagnostic accuracy of emergency physicians in interpreting 45 ECGs of patients with narrow-complex tachycardias, increasing the paper speed increased the physicians ability to make the correct diagnosis from 63% to 71% (P = 0.002).66 Irregularities and flutter waves are easier to appreciate with the paper speed doubled. Additionally, accelerating the paper speed resulted in fewer patients receiving inappropriate doses of adenosine.66
An inpatient or outpatient echocardiogram should be considered in patients with documented sustained SVT to exclude the possibility of structural heart disease. If tachycardia persists for weeks to months and results in a rapid ventricular rate, tachycardia mediated cardiomyopathy may develop and can be detected by echocardiogram.32
An ambulatory 24-hour Holter recording device can be useful in capturing abnormal rhythms in patient who present with frequent (i.e., several episodes per week) but transient tachycardias. An event monitor, which can be triggered by the patient when they feel the sensation of a tachydysrhythmia, is often more useful than a 24-hour Holter monitor in patients with less frequent arrhythmias.67-69 In a randomized crossover clinical trial of patients with palpitations, event monitors were twice as likely to provide a diagnostic rhythm strip electrocardiogram compared to 48-hour Holter monitoring (67% vs. 35%, P< 0.001).70 A cost-analysis of event monitors within this study demonstrated a $213-$373 cost saving compared to Holter monitoring. Patients discharged on these devices should be instructed to carry a journal with them and record the events, including meals and activities, surrounding the tachydysrhythmia.
The management of patients with symptoms suggestive of an arrhythmia but without ECG documentation depends on the nature of the symptoms. If the 12-lead ECG is normal and the patient reports a history consistent with premature extra beats, then precipitating factors (such as excessive caffeine, alcohol, nicotine, recreational drug use, hypokalemia, hypomagnesemia, or hyperthyroidism) should be reviewed and appropriately managed.
If the clinical history indicates that the dysrhythmia is paroxysmal in nature and the 12-lead ECG is normal, then a Holter or event monitor should be considered for the patient, along with outpatient cardiology consultation. This strategy is appropriate for patients who do not experience significant signs and symptoms associated with the dysrhythmia. Patients can be taught vagal maneuvers, and a β-blocking agent may be prescribed empirically provided that significant bradycardia (< 50 bpm) and hypotension (SBP < 90 mmHg) have been excluded. Due to the risk of development of proarrhythmias, antiarrhythmic treatment with Class I or Class III drugs should not be initiated without a documented dysrhythmia. Patients who report symptoms suggestive of compromised cardiovascular status, such as chest pain or syncope associated with a suspected transient tachydysrhythmia, require admission with telemetry monitoring and further inpatient cardiologic evaluation.
Sustained tachydysrhythmias that are present upon evaluation in the ED must be managed expeditiously. The initial approach and management of tachydysrhythmias consists of a rapid assessment and support of the patient's ABCs as well as placement of the patient on a cardiac monitor with automated BP cuff, continuous pulse oximetry monitoring, provision of supplemental oxygen as needed, and establishment of intravenous access. The emergency physician should attempt to identify and treat reversible causes of the tachycardia through appropriate history taking and selected laboratory analysis. A 12-lead ECG should be obtained as soon as possible; however, a rhythm strip may be all that is needed to initiate treatment.
The first decision point in determining therapy rests upon classification of the patient's clinical picture. If the patient demonstrates rate-related cardiovascular compromise with significant signs and symptoms (such as altered mental status, ongoing ischemic chest pain, hypotension, or other signs of shock), immediate synchronized cardioversion is indicated. Ventricular rates < 150 bpm are unlikely to be the proximate cause of instability in patients with healthy underlying myocardium. An exception to the immediate cardioversion rule occurs when IV access has been obtained, adenosine is available, and there is an anticipated delay to synchronized cardioversion. In this case, if a patient becomes unstable with a narrow-complex reentry SVT, adenosine (ACC/AHA Class IIb recommendation for reentry SVT)11 can be given immediately while preparations are being made for synchronized cardioversion. The general approach to the treatment of SVT rests upon slowing conduction through the SA and AV nodes. Most SVTs due to a reentry mechanism involve one of these two regions, and successful interruption of conduction through these nodes frequently terminates the SVT. Short acting therapies such as vagal maneuvers or adenosine can be used to temporarily interrupt SA and AV nodal conduction. Long-acting AV nodal blocking agents, such as nondihydropyridine calcium-channel blockers (i.e., verapamil, diltiazem) and β-blockers are appropriate for patients with SVT due to enhanced automaticity mechanisms. Table 4 provides a summary of ACC/ AHA recommendations for patients presenting with narrow-complex or wide-complex tachycardia.11

AV nodal blocking interventions include vagal maneuvers (e.g., carotid sinus massage, valsalva maneuver, cold-water facial immersion), adenosine, nonhydropyridine calcium-channel blockers, and β-blockers. All may be used with success in narrow-complex SVT.11
Massage of the carotid sinus, having the patient valsalva, and cold-water facial immersion have all been used with varying success in the treatment of SVT. In a controlled clinical trial of 148 patients, carotid sinus massage, valsalva maneuver, or the combination of carotid sinus massage and valsalva maneuver were successful in terminating nearly 30% of SVTs.71 A comparison of these techniques for termination of AVRT and AVNRT found that the valsalva maneuver in the supine position was the most effective, with 54% of SVTs successfully treated by this method. Termination rates were lower with carotid sinus massage (22%) and cold-water facial immersion (17%).72
It has been suggested that vagal maneuvers are most likely to be successful in terminating the SVT if initiated soon after the arrhythmia starts.73 Sympathetic tone often increases with prolonged duration of the tachydysrhythmias, rendering vagal maneuvers less effective. Patients should be educated regarding vagotonic methods and instructed to use these methods at the onset of symptoms.
Adenosine is an AV nodal blocking agent that can be used diagnostically to determine the underlying rhythm in patients with SVT and therapeutically to terminate certain supraventricular tachydysrhythmias.
Compared to other AV nodal blocking agents, adenosine has the advantages of having a short half-life (less than 10 seconds) and rapid onset of action; it is the preferred agent for conversion of reentry SVT (ACC/ AHA Class I recommendation).11The initial dose of adenosine is 6 mg rapid IV push. Adenosine must be given rapidly over 1-3 seconds through a large (e.g., antecubital) vein and then immediately followed by a 10-20 mL saline flush. If the rhythm does not convert within 1-2 minutes, a 12 mg rapid IV bolus should be given. A randomized double-blind placebo-controlled clinical trial involving 359 patients reported that approximately 60% of patients receiving adenosine converted to normal sinus rhythm after an initial 6 mg IV bolus.74 Failure to abolish reentry SVT with this dose is often related to pushing the medication too slowly and not immediately following the bolus with a saline flush. The subsequent 12 mg bolus is reported to be more than 90% effective.74,75 The initial and subsequent doses of adenosine should be reduced by 50% when pushed though a central line.
Five unique outcomes may occur with the administration of adenosine to a narrow-complex regular tachycardia. Adenosine may lead to 1) termination of the tachycardia, 2) gradual slowing then re-acceleration of the tachycardia, 3) demonstration of an atrial tachycardia with high grade AV block, 4) unmasking of a concealed accessory pathway, or 5) no effect. AVRT, AVNRT, and SNRT are typically terminated by adenosine. ST and NPJT gradually slow with adenosine and then reaccelerate. UAT infrequently terminates but may show a pattern of gradually slowing then reaccelerating with adenosine. AT, atrial flutter, and atrial fibrillation may demonstrate continued atrial tachydysrhythmia with high grade AV block. If no effect is seen with adenosine, the initial dose may have been inadequate or a narrow-complex VT may exist.
In a Cochrane Review of eight clinical trials, minor side effects occurred in 11% of patients receiving adenosine and included flushing, chest pain, dyspnea, headache, nausea and a "sense of doom."75These symptoms are transient (typically lasting 30-60 seconds in duration) and the patient should be warned prior to administration of the medication. Adenosine is proarrhythmic and both significant bradyarrhythmias and ventricular tachydysrhythmias have been reported to result from its administration.76
Adenosine should be used with caution in situations involving heart transplant patients and patients taking medications that may alter its effects (e.g., dipyridamole, carbamazepine, theophylline). Heart transplant patients are particularly sensitive to adenosine and the initial dose of therapy in this group of patients should be reduced to 1-3 mg.77 Adjusted adenosine dosing may be needed in the presence of 1) theophylline or caffeine, as they attenuate the effects of adenosine, 2) dipyridamole, as it potentiates the response to adenosine, and 3) carbamazepine, which may lead to higher rates of heart block.11 Adenosine is contraindicated in patients with atrial fibrillation with evidence of WPW syndrome.
Nonhydropyridine Calcium-Channel Blockers (ACC/AHA Class I recommendation for reentry SVT, Level of Evidence A) and β-Blockers (ACC/AHA Class IIb recommendation for reentry SVT, Level of Evidence C) The use of nondihydropyridine calcium channel blockers (i.e., verapamil, diltiazem) or β-blockers is appropriate for SVT patients that do not convert with adenosine.11
Diltiazem and verapamil act to slow conduction and increase refractoriness in the AV node. These actions may end reentrant arrhythmias and control ventricular rate in a variety of supraventricular tachydysrhythmias. Verapamil and, to a lesser extent, diltiazem can decrease blood pressure, although these effects may not be as pronounced when either agent is given as a slow continuous infusion78 or when pretreatment with calcium is provided.79 A review of six clinical trials involving 322 patients with supraventricular tachydysrhythmias, including SVT, MAT, and atrial fibrillation, demonstrated that pretreatment with calcium salts significantly attenuates or eliminates the expected drop in systolic blood pressure, but not the heart rate, in patients receiving verapamil.79
Diltiazem is dosed at 0.25 mg/kg IV and administered over two minutes. A subsequent dose of 0.35 mg/kg IV is given if no response is seen and in the absence of any significant drug-induced adverse vent. Verapamil is dosed at 2.5-5 mg IV. A follow-up dose of 5-10 mg IV is given if the SVT persists. A contemporary randomized trial of 161 hemodynamically stablepatients demonstrated equivalence between these two agents in successful termination of SVT (99% with verapamil,96% with diltiazem, P = NS), with recurrence rates of less than 5% during the two-hours following administration.78 A subgroup analysis of the above study involving 15 patients with initial systolic blood pressure less than 90 mmHg demonstrated 100% efficacy in terminating SVT when diltiazem or verapamil was given as a slow infusion at a rate of 1 mg/min for verapamil and 2.5 mg/min for diltiazem.78 Neither agent is recommended in the setting of impaired ventricular function or heart failure.11
β-adrenergic blocking agents decrease heart rate and blood pressure. A variety of agents (e.g., atenolol, metoprolol, propranolol, esmolol) may be used; however,side effects such as significant bradycardias, AV conduction delay, and hypotension may occur. Metoprolol is given as a 5 mg infusion over two minutes and can be repeated up to three doses in 15 minutes. Propranolol is given as an infusion of 0.15 mg/kg over a two minute period. Esmolol is given as a 250-500 mcg/kg bolus over one minute followed by a 50 mcg/ kg/min infusion with an increase in the infusion rate by 50 mcg/kg/min every 4 minutes until rate control or a maximum infusion rate of 200 mcg/kg/min are achieved. Contraindications to the use of these agents include second-degree or third-degree heart block, hypotension, severe congestive heart failure, and lung disease associated with bronchospasm. No EDbased trials comparing calcium-channel blockers and β-blockers for the acute termination of SVT have been performed; however, the results of one small ICU study suggested that diltiazem is superior to esmolol in this regard.80 These results parallel those from an ED-based trial comparing diltiazem and metoprolol in the treatment of atrial fibrillation.81
Both calcium-channel blockers and β-blockers are contraindicated in patients with atrial fibrillation/flutter and evidence of WPW syndrome.82 Administration of these agents can paradoxically increase conduction through the accessory pathway and precipitate rapid ventricular rates or ventricular fibrillation resulting incardiovascular collapse.83-85
Procainamide, ibutilide, flecainide, propafenone, and amiodarone can all be used to control the rhythm in atrial fibrillation/flutter in patients with preexcitation and WPW syndrome.11,17 Additionally, these medications can be used to treat reentry SVT when the rhythm remains uncontrolled despite vagal maneuvers, adenosine, and longer-acting AV nodal blocking agents.These agents should be used in conjunction with expert consultation.
Procainamide, a class IA antiarrhythmic, has been used to suppress supraventricular tachydysrhythmias by slowing electrical conduction through the heart by prolonging the refractory period of cardiac tissue including accessory pathways. Procainamide is administered at a rate of 20 mg/min until one of four outcomes occur: 1) arrhythmia suppression, 2) the QRS duration is prolonged more than 50% from its baseline, 3) hypotension develops, or 4) a maximum of 17-20 mg/kg has been administered. The maintenance infusion rate of procainamide is 1-4 mg/min. Procainamide should be used with caution in patients with preexisting QT prolongation.
Amiodarone is dosed as a 150 mg IV infusion over 10 minutes, followed by 1 mg/min infusion over six
hours, and then 0.5 mg/min maintenance over 18 hours. In patients with severely impaired heart function, IV amiodarone is preferable to other antiarrhythmic agents for supraventricular tachydysrhythmias. The major adverse effects of amiodarone in the acute setting are hypotension and bradycardia, which can be prevented by slowing the rate of drug infusion.
Cardioversion is recommended to treat the following cases of supraventricular tachydysrhythmias when the patient is unstable: 1) SVT due to reentry, and 2) atrial fibrillation or atrial flutter.11 Synchronized cardioversion is the preferred treatment for atrial fibrillation/ flutter in patients with WPW syndrome.11,30,86Delivery of a synchronized shock (i.e., timed to prevent shock delivery during cardiac repolarization) can stop these rhythms because it interrupts the circulating (reentry) pattern. The recommended initial dose for cardioversion of SVT is 50 J to 100 J with a monophasic defibrillator
and 30 J to 50 J with a biphasic defibrillator. While current recommendations endorse low-energy shocks, a recent study indicated that there is a small but significant increase in incidence of ventricular fibrillation after monophasic shock with less than 200 J compared to shock with more than 200 J.87
Cardioversion is not likely to be effective for treatment of junctional tachycardia or atrial tachycardia
(focal or multifocal). A paradoxical increase in heart rate may occur when synchronized electricity is given to a supraventricular tachydysrhythmia due to an enhanced automaticity mechanism.
If possible, IV access should be obtained for procedural sedation prior to cardioversion in the awake
patient. Etomidate and fentanyl are good choices for pre-medication due to their short onset of action, limited duration, and narrow effect on blood pressure.
Treatment of physiological sinus tachycardia consists of identifying the underlying etiology and addressing it. Amongst the common causes of ST seen in the ED, fever, hypovolemia, and anxiety should be addressed and appropriately managed. β-blockers may be useful for physiologic ST triggered by emotional stress and other anxiety-related disorders, for prognostic benefit after myocardial infarction and congestive heart failure, and for patients with thyrotoxicosis. Catheter ablation is a controversial option for patients with inappropriate ST that cannot be controlled with β-blocker or calciumchannel blockers.88-90
Sinoatrial Nodal Reentrant Tachycardia (SNRT) Sinoatrial nodal reentrant tachycardia is typically terminated by AV nodal blocking interventions. Electrophysiologic referral is reserved for patients
with frequent or poorly tolerated episodes of tachycardia that do not adequately respond to drug therapy. Catheter ablation of persistent SNRT is generally successful.91,92
Both the typical (slow-fast) and atypical (fast-slow or slow-slow) forms of AVNRT may be treated by
interruption of the reentry loop through AV nodal inhibition, by slowing conduction through the heart (e.g., amiodarone, procainamide), or with direct cardioversion.
The "pill in the pocket" approach is recommended for patients with infrequent, well tolerated, but prolonged episodes of AVNRT. Patients are given instructions to self administer an oral dose of an AV nodal blocking agent only after failure of vagal maneuvers to terminate the SVT. In one study of 33 patients, single dose therapy with diltiazem 120 mg and propranolol 80 mg was superior to flecainide and placebo in rate of conversion to sinus rhythm. Conversion to sinus rhythm occurred in 94% of the patients receiving the diltiazem/propranolol combination compared to 61% and 52%, respectively, in the flecainide and placebo groups.93 Additionally, the "pill in the pocket" approach in this trial was associated with a significant decrease in the number of subsequent emergency department visits. This approach is not appropriate for patients with ECG evidence of preexcitation, sinus bradycardia, or significant left ventricular dysfunction.
Chronic daily therapy is only needed if patients experience frequent or poorly tolerated episodes of AVNRT. First line therapies include nondihydropyridine calcium-channel blockers, β-blockers and digoxin. In the absence of structural heart disease, patients who do not respond to AV nodal blocking agents may be started on class Ic (flecainide, propafenone) antiarrhythmics. Class III (sotalol, amiodarone) antiarrhythmics are used infrequently. Catheter ablation is increasingly being recommended for symptomatic or sustained AVNRT.
The approach to AVRT depends upon the appearance of the ECG. A paroxysmal narrow-complex regular tachycardia due to an accessory pathway must be conducted orthodromically. Termination of this arrhythmia is identical to termination of any other paroxysmal narrow-complex regular tachycardia: start with vagal maneuvers and adenosine; then, if unsuccessful, move on to long-acting AV nodal blocking agents. Keep in mind that the use of adenosine to terminate this rhythm has been associated with the development of atrial fibrillation and its sequelae (rapid ventricular rates, cardiovascular compromise, and ventricular fibrillation) in patients with WPW syndrome.76,94
A paroxysmal wide-complex regular tachycardia due to an accessory pathway must be conducted
antidromically. One must always initially assume a paroxysmal wide-complex regular tachycardia is ventricular tachycardia and treat it as such. Procainamide or amiodarone may be used in the hemodynamically stable patient, whereas synchronized cardioversion is indicated for the unstable patient.
A paroxysmal wide-complex irregular tachycardia due to an accessory pathway can only be due to
atrial fibrillation or variably conducted atrial flutter with preexcitation. Synchronized cardioversion is the
preferred treatment for this situation, although procainamide or ibutilide may be tried first if the patient
is hemodynamically stable. Short-acting or long-acting AV nodal blocking agents are contraindicated and must be avoided.
Catheter ablation is becoming an increasingly popular option for the long-term management of patients
with an accessory pathway mediated tachycardia. Long-acting AV nodal blocking agents and Class Ic or
III antiarrhythmic medications may be used with some success in patients not desiring catheter ablation or as bridging therapy until catheter ablation is performed.
The response of UAT to medication or electricity is dependent upon the mechanism responsible for its
genesis. A significant proportion of UAT that is due to micro-reentry or triggered activity is sensitive to
AV nodal blocking interventions and may be successfully terminated by DC cardioversion. These patients display either termination of the arrhythmia or tachycardia persistence with high-degree AV block upon administration of AV nodal blocking medications. For patients with automatic AT, adenosine may result in transient slowing without termination of the dysrhythmia, and DC cardioversion is rarely effective. Unless electrophysiologic studies have been performed, the mechanism of UAT is rarely established, making it difficult to tailor treatment. Moreover, UAT is often challenging to manage medically. Class Ia, Ic, or III antiarrhythmics as well as synchronized cardioversion
may be indicated if AV nodal agents are unsuccessful at rhythm termination.
UAT, typically with AV block, can be seen with digoxin poisoning. Treatment in this specific instance
consists of discontinuation of digoxin. If high grade AV block persists, digitalis-binding agents should
be considered.
Pooled data from 514 patients who underwent catheter ablation for focal AT showed an 86% success
rate with an 8% recurrence rate.11,95Recurrence is often due to a second or third irritable focus within the atrium that becomes the new dominant focus once the previous dominant focus has been ablated. Patients may require ablation of several irritable foci within the atria. A recent case series demonstrated a 96% success rate when up to three foci were ablated in patients with UAT.96
Little is known about the response of PJT to suppressive drug therapy; however, long-acting AV nodal
blocking agents are theorized to be effective and antiarrhythmic therapy with Class Ia, Ic, or III medications has been used in refractory cases.97-100 Definitive therapy is obtained via catheter ablation, though this carries a 5-10% risk of permanent AV nodal block.101,102
The management strategy with NPJT focuses on correction of the underlying abnormality. When NPJT is due to digitalis toxicity, withholding digitalis may be all that is required to return to a sinus rhythm. Digitalis-binding agents should be used if ventricular arrhythmias or high grade AV block is evident.40 Other conditions, such as hypokalemia, chronic obstructive pulmonary disease (COPD), myocardial ischemia, and myocarditis should be appropriately managed. Longacting AV nodal blocking agents are effective in cases of persistent NPJT.
Therapy for MAT is directed at correction of the underlying precipitant. Acute decompensation from a chronic pulmonary disease and correction of hypoxia should be addressed when present. Similarly, correction of electrolyte abnormalities (hypokalemia, hypomagnesemia) and drug toxicity (sympathomimetics, digoxin, theophylline) may help suppress the nondominant atrial pacemakers present in MAT. Chronic therapy may employ the use of long-acting AV nodal blocking agents; 103 however, β-blocking agents should be used with caution in patients with severe underlying pulmonary disease.11 One study involving only 13 patients has questioned this conventional wisdom and found metoprolol to be more effective than verapamil in reducing ventricular rate and achieving conversion to sinus rhythm.103 There is no role for DC cardioversion, antiarrhythmic drugs, or catheter ablation with MAT.
The goal of electrophysiologic testing is to perform cardiac mapping that identifies the origin or a critical site in the conduction path of a dysrhythmia. Once identified, catheter ablation of the site of origin or path of propagation can be performed. The success and complication rates of these procedures vary with the type of SVT. All SVT patients with severe symptoms (e.g., syncope), those with drug resistance or intolerance, or those who prefer to be free of drug therapy, should be referred for electrophysiologic evaluation.104 Given he uncertainty in establishing a definitive diagnosis in patients with wide-QRS complex tachycardia, some authors have recommended that all patients with widecomplex tachycardias be referred for electrophysiologic testing and evaluation. Studies have shown multiple advantages to catheter ablation, including a higher success rate than with antiarrhythmic medications, lower long-term cost than antiarrhythmic medications, and improved quality of life.105 The incidence of significant complications is low (0-2%) in experienced centers, but includes cardiac perforation, phrenic nerve injury, sinus node dysfunction, and AV node dysfunction.104
Patients with symptomatic preexcitation syndromes should be referred for cardiology and electrophysiologic evaluation. The utility of routine referral for asymptomatic preexcitation syndrome is currently an area of controversy.
A paroxysmal wide-complex regular tachycardia without clear P waves preceding the QRS complex at a fixed interval should be assumed to be ventricular tachycardia (VT) (Figure 13) until proven otherwise. Only when the QRS morphology during SVT is unchanged from QRS morphology during sinus rhythm should the diagnosis of SVT with BBB be entertained in the ED (Figures 14 and 15). Caution is mandatory as there are case reports of confirmed VT where the QRS morphology appeared identical to the QRS morphology during sinus rhythm.106,107 A wide-QRS tachycardia may also be seen with antidromic AVRT, hyperkalemia, and significant sodium channel blockade (e.g., tricyclic antidepressants, diphenhydramine, cocaine).108 Sinus tachycardia with a BBB (Figure 16) and atrial flutter with a BBB should be eliminated from the differential based on the location and appearance of the P wave.




Although certain demographic and electrocardiographic features make the diagnosis of VT more likely, the presence of normal vital signs is not helpful. Generally, patients more than 50 years of age and those with a history of myocardial infarction or congestive heart failure are likely to present with VT. SVT with a BBB or antidromic AVRT may be more likely diagnoses in patients less than 35 years of age with a history of SVT, but a wide-complex regular tachycardia should nonetheless be presumptively managed as VT.
Several ECG criteria have been described to differentiate the underlying mechanism of wide-QRS tachycardia. Ventricular atrial dissociation with a ventricular rate faster than the atrial rate generally proves the diagnosis of VT but is clearly discernable in only 30% of all VTs.109 The presence of fusion complexes is uncommon but also favors the diagnosis of VT. Fusion complexes represent a merger between conducted sinus or supraventricular impulses and ventricular depolarization occurring during AV dissociation. Additionally, an RS (from the initial R to the nadir of S) interval longer than
100 msec in any precordial lead is suggestive of VT.
A QRS width of more than 0.14 seconds with a RBBB configuration or 0.16 seconds with a LBBB configuration favors VT but is not helpful in differentiating VT from antidromic AVRT. Finally, a QRS pattern with negative concordance in the precordial leads suggests VT. Negative concordance occurs when all the QRS patterns in the precordial leads are similar and appear as QS complexes (Figure 13). Despite ECG criteria, patients presenting with wide-QRS tachycardia are often misdiagnosed.110,111 Furthermore, no ECG criteria is foolproof; exceptions occur and are documented in the literature.112, 113
The safest approach to these tachydysrhythmias is to treat them presumptively as VT. Synchronized direct current (DC) cardioversion (ACC/AHA Class I recommendation, Level of Evidence B) is indicated if the patient is unstable.11 Antiarrhythmic therapy with procainamide, sotalol, or amiodarone (each considered ACC/AHA Class I recommendations, Level of Evidence B) is appropriate if the patient is stable.11 Varying efficacies have been reported for these agents.114-117 In a recent small retrospective study, amiodarone was successful in terminating VT in only 29% of patients.114 Further studies investigating the efficacy of amiodarone in terminating VT are needed. However, amiodarone is still the preferred antiarrhythmic agent in the setting of poor LV function. The use of longacting AV nodal blocking agents (ACC/AHA Class III recommendation, Level of Evidence B) can precipitate hemodynamic collapse in VT and should be avoided. 11,118 Expert consultation is recommended to assist in the diagnosis of these patients.
A paroxysmal wide-complex irregular tachycardia is most likely to be atrial fibrillation with BBB (Figure 17); however, MAT and variable atrial flutter with a BBB may also present this way. Characterization and identification of the P waves should allow for differentiation between these entities. Non-supraventricular rhythms are to be considered, especially ventricular fibrillation and polymorphic ventricular tachycardia (Figure 18). Finally, atrial fibrillation in the presenceof accessory pathway conduction (Figure 8) is a widecomplex, irregular tachycardia and is discussed below.

The classic ECG findings in WPW syndrome are: 1) shortened PR interval, 2) initial upslurring of the QRS complex, referred to as the δ wave, and 3) borderline wide or wide-QRS complex (Figure 5). While orthodromic and antidromic AVRT are the most common arrhythmias in WPW syndrome, atrial fibrillation has been documented to occur in up to 30% of patients and can be life threatening.119 During atrial fibrillation, atrial impulses can be quickly conducted via the accessory pathway, producing extremely high ventricular rates with potential for rapid deterioration into ventricular fibrillation.85,120 Although this occurs uncommonly and sudden cardiac death is rare in WPW syndrome,121 these deaths are preventable with ablation of the accessory pathway.
Emergency physicians must know how to treat atrial fibrillation/flutter in the setting of WPW syndrome. A good memory aid to remember the medications that are contraindicated is "no ABCD drugs," where the "A" represents adenosine, the "B" represents β-blockers, the "C" represents calcium-channel blockers, and the "D" represents digoxin. Unstable patients with preexcited atrial fibrillation should undergo immediate synchronized cardioversion. In stable patients, synchronized cardioversion (ACC/ AHA Class I recommendation, Level of Evidence C) is a safe option;11 however, antiarrhythmic medications that slow conduction via the accessory pathway may be used.122,123Appropriate choices include intravenous procainamide, amiodarone, flecainide, or ibutilide (each considered ACC/AHA Class I recommendations, Level of Evidence B).11 A recent review article detailing the medical literature regarding amiodarone in this setting calls into question its efficacy and safety profile. The authors conclude that DC cardioversion represents the safest option for these patients.30,86
It is rare for women to have a first occurrence of SVT during pregnancy. However, in women with a prior history of SVT, more frequent episodes of SVT may occur during pregnancy.124 As in the general population, the most common forms of SVT in pregnant women are AVNRT and AVRT. It is unusual for an SVT to significantly compromise maternal or fetal health. However, at extremes of heart rates or in the presence of underlying cardiac disease, severe symptoms and hemodynamic instability may occur.
All commonly used antiarrhythmic drugs cross the placenta. Consequently, pharmacologic treatment of SVT in pregnant females should be avoided when possible, especially during the first trimester. First line therapy should consist of vagal maneuvers and then medications with the longest safety record.125-127
Management of SVT in pregnancy does not differ significantly from the approach in the non-pregnant patient. In hemodynamically unstable patients, electrical cardioversion is indicated and is well tolerated (ACC/ AHA Class I recommendation, Level of Evidence C).11 In the stable patient with SVT, vagal maneuvers (ACC/ AHA Class I recommendation, Level of Evidence C) should be employed first and, if unsuccessful, should be followed by adenosine (ACC/AHA Class I recommendation, Level of Evidence C).11,128-130 β-blockers are generally considered safe in pregnancy (ACC/AHA Class IIa recommendation, Level of Evidence C).11 Cardioselective β-1 blockers (metoprolol) are preferred due to the theoretical advantage of less interference with β-2 mediated peripheral vasodilatation and uterine relaxation.131 Both verapamil and diltiazem have been used in pregnancy to successfully treat SVT (ACC/AHA Class IIb recommendation, Level of Evidence C).11,132,133 For atrial fibrillation or flutter in WPW syndrome and wide complex tachycardias of unknown etiology, procainamide is the treatment of choice in pregnancy. Amiodarone is not considered safe during pregnancy due to multiple adverse effects on the fetus; it should only be used for life threatening conditions refractory to other treatments after careful consideration of risks and benefits.134-136 Other medications considered safe in pregnancy for treatment of SVT include digoxin, flecainide, and quinidine.137,138
SVT is the most common symptomatic arrhythmia requiring medical attention in the pediatric population.139 SVT should be suspected in any child or adolescent with a heart rate exceeding 180 bpm and any infant with a heart rate greater than 220 bpm.
The initial treatment in stable patients is vagal maneuvers. A modified vagal maneuver (such as blowing into an obstructed straw) can be used in younger children. Cold-water facial immersion (the diving reflex) has been used in children with varying success.139-142 Adenosine is used if vagal maneuvers do not break the SVT.143 In children, the initial adenosine dose is 0.1 mg/kg rapid IV push (maximum dose = 6 mg). Subsequent doses are increased by 0.1 mg/kg to a maximum of 0.3 mg/kg (maximum dose = 12 mg). In stable patients who do not respond to adenosine, antiarrhythmic medications (procainamide, esmolol, amiodarone) may be useful and should be considered in consultation with a pediatric cardiologist.144,145Synchronized cardioversion is performed in unstable patients or patients with refractory SVT. The initial energy dose is 0.5-1 Joule/kg, and subsequent doses can be doubled to a maximum of 2 Joules/kg
There is a lack of large-scale randomized trials comparing long-term outcomes in patients receiving catheter ablation for SVT. Limited data suggest that, as compared to antiarrhythmic therapy, catheter ablation improves quality of life and is more cost-effective in the long term.105,146
Although the incidence of sudden cardiac death due to rapid conduction of atrial fibrillation that leads to ventricular fibrillation is expected to be between 0.15% and 0.45% per patient year over a 3-10 year follow-up,147-150 the optimal treatment for patients with asymptomatic preexcitation syndrome has yet to be established.120 Using data obtained from invasive and noninvasive electrophysiologic studies, attempts have been made to stratify patients with preexcitation syndrome into groups at higher risk for progression to sudden cardiac death.151-154 Two recent trials, one involving pediatric patients and the other involving adult patients with WPW syndrome at high-risk for arrhythmias, demonstrated a substantial risk reduction in the development of cardiac arrhythmias following catheter ablation therapy.155,156
There are no established guidelines and little research investigation regarding disposition decisions for patients with SVT. A single-center, retrospective study among patients who presented to the ED with SVT found that 71% were discharged after an average stay of four hours.157 The 24-hour recurrence rate was less than 5% in patients discharged from the ED. Furthermore, there were no deaths recorded in admitted or discharged patients. Data from the National Hospital AmbulatoryMedical Care Survey 1993-2003 database revealed a 76% discharge rate for SVT after evaluation in the ED.1
In ST, MAT, and NPJT, there may be serious underlying disease that precipitates the onset of SVT. In these instances, admission to the hospital may be required to treat comorbid conditions and stabilize the patient.
In general, most people with lone SVT can be discharged home after a short observation period if therapy in the ED results in prompt conversion to sinus rhythm. A sustained SVT with the need for ongoing monitoring and treatment is an indication for admission. Hospitalization is also recommended for patients with significant symptoms (e.g., syncope, angina, severe hypotension) associated with the SVT. One study in the pediatric population suggested admission for patients under 3 months of age or with immediate recurrence of SVT after treatment due to a higher probability of recurrence and negative outcome in these patients.158 Referral to a cardiologist with prompt follow-up should be made in patients with WPW syndrome associated symptomatic arrhythmias.
Supraventricular tachycardia is a common dysrhythmia in the adult and pediatric population. While SVT
is rarely life threatening, it can result in hemodynamic instability that requires immediate electrical cardioversion. Using a structured approach to determine the QRS width, the RR regularity and the P wave characteristics of the underlying rhythm can aid in determining the ECG diagnosis of patients with SVT. Although the majority of SVT can be terminated with AV nodal blocking agents or maneuvers, antiarrhythmic medication and DC cardioversion may need to be employed. Patients with atrial fibrillation and WPW syndrome need to be urgently identified and treated with DC cardioversion (preferred) or appropriate antiarrhythmic therapy.
1. "I have a lot of experience differentiating SVT with aberrancy from VT - I'm really on top of the various criteria you can use to tell one from the other."
2. "I can't believe that patient submitted a complaint. Adenosine was the indicated treatment for his SVT and I administered it right after he rolled in the door."
3. "That lady had "psych" written all over her - a history of depression, anxiety, and requent ED visits for palpitations."
4. "Young people can tolerate rapid heart rates; I never use electrical cardioversion ecause those young whipper-snappers never seem unstable -- a blood pressure of 95/50 is normal for them."
5. "The elderly gentleman with a history of myocardial infarction (MI) had shortness of breath and an irregular rhythm on ECG with a rate of 120. I knew he wouldn't tolerate a heart rate of 120 for very long so I administered metoprolol for rate control."
6. "It was a really busy shift; she was a healthy young woman who was just coming in for a refill on her allergy medication. She was tachycardic in triage, but didn't mention any specific complaints."
7. "I wasn't sure what to do with that kid…he was 4-years-old with a history of Ebstein's anomaly and heart failure. He was slightly tachypneic with a blood pressure on the low-normal side. His ECG was difficult to interpret but it looked like he was in a preexcited SVT."
8. "It was a regular narrow complex tachycardia on the monitor and I couldn't see any P waves. While the nurse was pulling adenosine, I figured it couldn't hurt to try carotid sinus massage."
9. "The young woman had a history of SVT and presented with her usual symptoms of palpitations and lightheadedness. When I spoke with her cardiologist, he recommended amiodarone; the first dose was given in the ED."
10. "The elderly diabetic woman was on digoxin for previously diagnosed atrial fibrillation. Today she presented with fatigue. Her ECG demonstrated NPJT and I sent a digoxin level which came back mildly elevated. I admitted her to the hospital for close monitoring."
You prepared the discharge paperwork for the anxious 35-year-old female and asked the nurse to send her home with primary care physician follow-up. The patient was walking towards the exit when the nurse handed you the ECG you requested for this patient. The ECG demonstrated what you had expected: sinus rhythm. However, the QRS complex caught your eye and you noted a delta wave. Luckily, the patient hadn't left the ED yet. After an informed discussion with the patient regarding her new diagnosis of WPW syndrome, she was discharged home with a follow-up cardiology appointment within the next week and instructions to follow-up with an electrophysiologic specialist.
You used synchronized cardioversion to treat the SVT in the older male presenting with intermittent chest pain and shortness of breath. You thought about using adenosine, but his declining mental status was concerning enough to classify his SVT as unstable. He converted to sinus rhythm with a rate of 90 bpm. Looking back at his records from prior visits, you realized his blood pressure had always been around 170/90. It seems very clear now that his blood pressure of 100/50 today was producing mental status changes and you were glad that you made the call to cardiovert rather than use adenosine.






















Evidence-based medicine requires a critical appraisal of the literature based upon study methodology andnumber of subjects. Not all references are equally robust. The findings of a large, prospective, randomized, and blinded trial should carry more weight than a case report.
To help the reader judge the strength of each reference, pertinent information about the study, such as the type of study and the number of patients in the study, will be included in bold type following the reference, where available. In addition, the most informative references cited in this paper, as determined by the authors, will be noted by an asterisk (*) next to the number of the reference.
Jennifer Carnell; Amandeep Singh
April 1, 2008